Polymers
-
 Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 9821
Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 9821 -
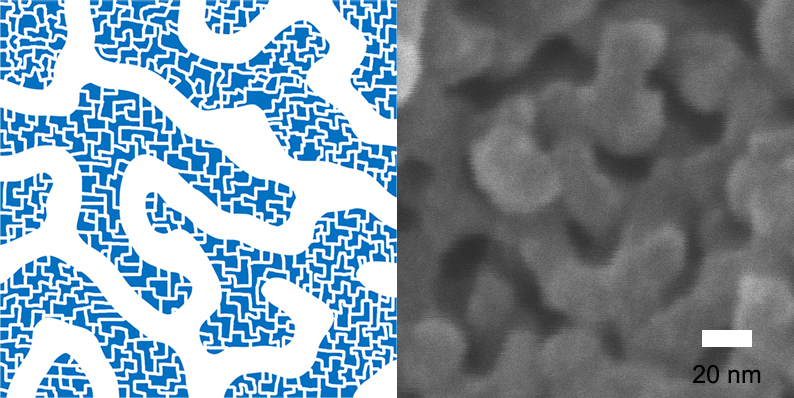 Hierarchically-Porous Polymers with Fast Absorption
KAIST's Professor Myungeun Seo and his research team from the Graduate School of Nanoscience and Technology has developed a method to form micropores of less than 2 nanometers within porous polymers where 10 nanometers long mesopores connect like a net. The advantage of the porous polymers is fast absorption of molecules.
Porous polymers with micropores of less than 2 nanometers, like a zeolite, have a large surface area. They are used as a means to store hydrogen-based molecules or as a catalytic support that can be used as a surface to convert a material into a desired form. However, because the size of the pores in its path was too small for the molecules, it took a long time to spread into the pores and reach the surface.
To reach the surface efficiently, a lung cell or the vein of a leaf has a structure wherein the pores are subdivided into different sizes so that the molecule can spread throughout the organ. A technology that can create not only micropores but also bigger pores was necessary in order to create such structure.
The research team solved the issue by implementing a "self-assembly" of block polymers to easily form a net-like nanostructure from mesopores of 10 nanometers.
The team created hierarchically-porous polymers consisting of two different types of pores by using a hypercrosslinking reaction along with the "self-assembly" method. The reaction creates micropores within the chain after the polymer chain is confined by a chemical bond.
This porous polymer has micropores that are smaller than 2 nanometers on the walls of mesopores while 10 nanometers long mesopores forming 3-dimensional net structures. Because of the "self-assembly" method, the size of mesopores can be adjusted within the range of 6 to 15 nanometers.
This is the first case where a porous polymer has both well-defined mesopores and micropores. The research team verified the effect of hierarchically-porous structures on absorption of molecules by confirming that the porous polymer had faster absorption speeds than a polymer consisting only of micropores.
Professor Seo said, “The study has found a simple way to create different sizes of pores within a polymer.” He expected that the hierarchically-porous polymers can be used as a catalytic support in which fast diffusion of molecules is essential, or for molecule collection.
The research was sponsored by National Research Foundation of Korea and published online in the Journal of the American Chemical Society.
Figure 1 – Net-like Structure of Hierarchically-Porous Polymers with Mesopores and Micropores on the walls of Mesopores.
Figure 2 - Hierarchically-Porous Polymers
Figure 3 – Comparison of Porous-Polymers consisting of Mesopores only (left), and Mesopores and Micropores (right)
2015.01.13 View 8000
Hierarchically-Porous Polymers with Fast Absorption
KAIST's Professor Myungeun Seo and his research team from the Graduate School of Nanoscience and Technology has developed a method to form micropores of less than 2 nanometers within porous polymers where 10 nanometers long mesopores connect like a net. The advantage of the porous polymers is fast absorption of molecules.
Porous polymers with micropores of less than 2 nanometers, like a zeolite, have a large surface area. They are used as a means to store hydrogen-based molecules or as a catalytic support that can be used as a surface to convert a material into a desired form. However, because the size of the pores in its path was too small for the molecules, it took a long time to spread into the pores and reach the surface.
To reach the surface efficiently, a lung cell or the vein of a leaf has a structure wherein the pores are subdivided into different sizes so that the molecule can spread throughout the organ. A technology that can create not only micropores but also bigger pores was necessary in order to create such structure.
The research team solved the issue by implementing a "self-assembly" of block polymers to easily form a net-like nanostructure from mesopores of 10 nanometers.
The team created hierarchically-porous polymers consisting of two different types of pores by using a hypercrosslinking reaction along with the "self-assembly" method. The reaction creates micropores within the chain after the polymer chain is confined by a chemical bond.
This porous polymer has micropores that are smaller than 2 nanometers on the walls of mesopores while 10 nanometers long mesopores forming 3-dimensional net structures. Because of the "self-assembly" method, the size of mesopores can be adjusted within the range of 6 to 15 nanometers.
This is the first case where a porous polymer has both well-defined mesopores and micropores. The research team verified the effect of hierarchically-porous structures on absorption of molecules by confirming that the porous polymer had faster absorption speeds than a polymer consisting only of micropores.
Professor Seo said, “The study has found a simple way to create different sizes of pores within a polymer.” He expected that the hierarchically-porous polymers can be used as a catalytic support in which fast diffusion of molecules is essential, or for molecule collection.
The research was sponsored by National Research Foundation of Korea and published online in the Journal of the American Chemical Society.
Figure 1 – Net-like Structure of Hierarchically-Porous Polymers with Mesopores and Micropores on the walls of Mesopores.
Figure 2 - Hierarchically-Porous Polymers
Figure 3 – Comparison of Porous-Polymers consisting of Mesopores only (left), and Mesopores and Micropores (right)
2015.01.13 View 8000