OT
-
 Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim>
"Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
*Chemical Synthesis: A process of creating desired substances using chemical reactions.
KAIST (President Kwang Hyung Lee) announced on the 31st of July that a research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances 'herpotrichones A, B, and C' for the first time.
Herpotrichone natural products are substances obtainable only in minute quantities from 'Herpotrichia sp. SF09', a symbiotic pill bug fungus, and possess a unique 6/6/6/6/3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).
Interestingly, this substance exhibits excellent anti-neuroinflammatory effects that suppress brain inflammatory reactions. Recently, its mechanism of action to protect nerve cells by inhibiting ferroptosis (iron-mediated cell death) was also reported, raising expectations for its potential as a therapeutic drug for brain diseases.
Professor Han's research team devised a biosynthetically inspired strategy to chemically synthesize herpotrichoneS. The key to success was a named chemical reaction "Diels-Alder (DA) reaction". This reaction forms a six-membered ring by creating new bonds between carbon-based partners, much like two puzzle pieces interlocking to form a single ring.
<Figure 2. Key Synthetic Strategy for Hypotricon A, B, and C Based on Hydrogen Bonding>
Furthermore, the research team focused on a weak attractive phenomenon between molecules called "hydrogen bonding". By delicately designing and controlling this hydrogen bond, they were able to precisely induce the reaction to occur chemo-, regio- and stereoselectively, thereby synthesizing herpotrichone. Notably, without the pivotal hydrogen bond, only a small amount of the target natural product was formed or only undesirable byproducts were generated.
The configuration of the C2’ hydroxyl moiety was essential in directing the desired transition states leading to the target natural products.
Thanks to this induced hydrogen bonding, the reacting molecules approached the correct positions and went through an ideal transition state, allowing for the synthesis of herpotrichone C. This reaction principle was also successfully applied to herpotrichone A and B, enabling the successful synthesis of these natural products.
During the key Diels-Alder reaction conducted in the laboratory, new molecular structures not yet discovered in nature were also formed. Some of these have a high probability of being novel natural products with excellent pharmacological activity, thus doubling the significance of this research for anticipating natural products through synthesis.
Indeed, while Professor Han's research team conducted synthetic studies on herpotrichone A and B based on a 2019 paper by Chinese researchers who discovered and elucidated their structures, the research team observed the formation of undesired byproducts.
Interestingly, in 2024, the same Chinese research team that discovered herpotrichones A and bn reported the discovery of a new natural product called herpotrichone C, which turned out to be the same substance as the major byproduct previously obtained by Professor Han's team en route to herpotrichones A and B.
Professor Han stated, "This is the first total synthesis of a rare natural product with pharmacological activity related to neurodegenerative diseases and systematically presents the principle of biomimetic synthesis of complex natural products." He added, "It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products."
This research outcome, with Yoojin Lee, a master's and Ph.D. integrated course student in the Department of Chemistry, as the first author, was published on July 16th in the Journal of the American Chemical Society (JACS), one of the most prestigious academic journals in the field of chemistry.
This research was supported by the National Research Foundation of Korea (NRF) Mid-career Researcher Support Program, the KAIST UP Project, the KAIST Grand Challenge 30 Project, and the KAIST Trans-Generational Collaborative Research Laboratory Project.
2025.08.04 View 229
Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim>
"Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
*Chemical Synthesis: A process of creating desired substances using chemical reactions.
KAIST (President Kwang Hyung Lee) announced on the 31st of July that a research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances 'herpotrichones A, B, and C' for the first time.
Herpotrichone natural products are substances obtainable only in minute quantities from 'Herpotrichia sp. SF09', a symbiotic pill bug fungus, and possess a unique 6/6/6/6/3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).
Interestingly, this substance exhibits excellent anti-neuroinflammatory effects that suppress brain inflammatory reactions. Recently, its mechanism of action to protect nerve cells by inhibiting ferroptosis (iron-mediated cell death) was also reported, raising expectations for its potential as a therapeutic drug for brain diseases.
Professor Han's research team devised a biosynthetically inspired strategy to chemically synthesize herpotrichoneS. The key to success was a named chemical reaction "Diels-Alder (DA) reaction". This reaction forms a six-membered ring by creating new bonds between carbon-based partners, much like two puzzle pieces interlocking to form a single ring.
<Figure 2. Key Synthetic Strategy for Hypotricon A, B, and C Based on Hydrogen Bonding>
Furthermore, the research team focused on a weak attractive phenomenon between molecules called "hydrogen bonding". By delicately designing and controlling this hydrogen bond, they were able to precisely induce the reaction to occur chemo-, regio- and stereoselectively, thereby synthesizing herpotrichone. Notably, without the pivotal hydrogen bond, only a small amount of the target natural product was formed or only undesirable byproducts were generated.
The configuration of the C2’ hydroxyl moiety was essential in directing the desired transition states leading to the target natural products.
Thanks to this induced hydrogen bonding, the reacting molecules approached the correct positions and went through an ideal transition state, allowing for the synthesis of herpotrichone C. This reaction principle was also successfully applied to herpotrichone A and B, enabling the successful synthesis of these natural products.
During the key Diels-Alder reaction conducted in the laboratory, new molecular structures not yet discovered in nature were also formed. Some of these have a high probability of being novel natural products with excellent pharmacological activity, thus doubling the significance of this research for anticipating natural products through synthesis.
Indeed, while Professor Han's research team conducted synthetic studies on herpotrichone A and B based on a 2019 paper by Chinese researchers who discovered and elucidated their structures, the research team observed the formation of undesired byproducts.
Interestingly, in 2024, the same Chinese research team that discovered herpotrichones A and bn reported the discovery of a new natural product called herpotrichone C, which turned out to be the same substance as the major byproduct previously obtained by Professor Han's team en route to herpotrichones A and B.
Professor Han stated, "This is the first total synthesis of a rare natural product with pharmacological activity related to neurodegenerative diseases and systematically presents the principle of biomimetic synthesis of complex natural products." He added, "It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products."
This research outcome, with Yoojin Lee, a master's and Ph.D. integrated course student in the Department of Chemistry, as the first author, was published on July 16th in the Journal of the American Chemical Society (JACS), one of the most prestigious academic journals in the field of chemistry.
This research was supported by the National Research Foundation of Korea (NRF) Mid-career Researcher Support Program, the KAIST UP Project, the KAIST Grand Challenge 30 Project, and the KAIST Trans-Generational Collaborative Research Laboratory Project.
2025.08.04 View 229 -
 Approaches to Human-Robot Interaction Using Biosignals
<(From left) Dr. Hwa-young Jeong, Professor Kyung-seo Park, Dr. Yoon-tae Jeong, Dr. Ji-hoon Seo, Professor Min-kyu Je, Professor Jung Kim >
A joint research team led by Professor Jung Kim of KAIST Department of Mechanical Engineering and Professor Min-kyu Je of the Department of Electrical and Electronic Engineering recently published a review paper on the latest trends and advancements in intuitive Human-Robot Interaction (HRI) using bio-potential and bio-impedance in the internationally renowned academic journal 'Nature Reviews Electrical Engineering'.
This review paper is the result of a collaborative effort by Dr. Kyung-seo Park (DGIST, co-first author), Dr. Hwa-young Jeong (EPFL, co-first author), Dr. Yoon-tae Jeong (IMEC), and Dr. Ji-hoon Seo (UCSD), all doctoral graduates from the two laboratories. Nature Reviews Electrical Engineering is a review specialized journal in the field of electrical, electronic, and artificial intelligence technology, newly launched by Nature Publishing Group last year. It is known to invite world-renowned scholars in the field through strict selection criteria. Professor Jung Kim's research team's paper, titled "Using bio-potential and bio-impedance for intuitive human-robot interaction," was published on July 18, 2025. (DOI: https://doi.org/10.1038/s44287-025-00191-5)
This review paper explains how biosignals can be used to quickly and accurately detect movement intentions and introduces advancements in movement prediction technology based on neural signals and muscle activity. It also focuses on the crucial role of integrated circuits (ICs) in maximizing low-noise performance and energy efficiency in biosignal sensing, covering thelatest development trends in low-noise, low-power designs for accurately measuring bio-potential and impedance signals.
The review emphasizes the importance of hybrid and multi-modal sensing approaches, presenting the possibility of building robust, intuitive, and scalable HRI systems. The research team stressed that collaboration between sensor and IC design fields is essential for the practical application of biosignal-based HRI systems and stated that interdisciplinary collaboration will play a significant role in the development of next-generation HRI technology. Dr. Hwa-young Jeong, a co-first author of the paper, presented the potential of bio-potential and impedance signals to make human-robot interaction more intuitive and efficient, predicting that it will make significant contributions to the development of HRI technologies such as rehabilitation robots and robotic prostheses using biosignals in the future. This research was supported by several research projects, including the Human Plus Project of the National Research Foundation of Korea.
2025.07.24 View 356
Approaches to Human-Robot Interaction Using Biosignals
<(From left) Dr. Hwa-young Jeong, Professor Kyung-seo Park, Dr. Yoon-tae Jeong, Dr. Ji-hoon Seo, Professor Min-kyu Je, Professor Jung Kim >
A joint research team led by Professor Jung Kim of KAIST Department of Mechanical Engineering and Professor Min-kyu Je of the Department of Electrical and Electronic Engineering recently published a review paper on the latest trends and advancements in intuitive Human-Robot Interaction (HRI) using bio-potential and bio-impedance in the internationally renowned academic journal 'Nature Reviews Electrical Engineering'.
This review paper is the result of a collaborative effort by Dr. Kyung-seo Park (DGIST, co-first author), Dr. Hwa-young Jeong (EPFL, co-first author), Dr. Yoon-tae Jeong (IMEC), and Dr. Ji-hoon Seo (UCSD), all doctoral graduates from the two laboratories. Nature Reviews Electrical Engineering is a review specialized journal in the field of electrical, electronic, and artificial intelligence technology, newly launched by Nature Publishing Group last year. It is known to invite world-renowned scholars in the field through strict selection criteria. Professor Jung Kim's research team's paper, titled "Using bio-potential and bio-impedance for intuitive human-robot interaction," was published on July 18, 2025. (DOI: https://doi.org/10.1038/s44287-025-00191-5)
This review paper explains how biosignals can be used to quickly and accurately detect movement intentions and introduces advancements in movement prediction technology based on neural signals and muscle activity. It also focuses on the crucial role of integrated circuits (ICs) in maximizing low-noise performance and energy efficiency in biosignal sensing, covering thelatest development trends in low-noise, low-power designs for accurately measuring bio-potential and impedance signals.
The review emphasizes the importance of hybrid and multi-modal sensing approaches, presenting the possibility of building robust, intuitive, and scalable HRI systems. The research team stressed that collaboration between sensor and IC design fields is essential for the practical application of biosignal-based HRI systems and stated that interdisciplinary collaboration will play a significant role in the development of next-generation HRI technology. Dr. Hwa-young Jeong, a co-first author of the paper, presented the potential of bio-potential and impedance signals to make human-robot interaction more intuitive and efficient, predicting that it will make significant contributions to the development of HRI technologies such as rehabilitation robots and robotic prostheses using biosignals in the future. This research was supported by several research projects, including the Human Plus Project of the National Research Foundation of Korea.
2025.07.24 View 356 -
 KAIST Team Develops Optogenetic Platform for Spatiotemporal Control of Protein and mRNA Storage and Release
<Dr. Chaeyeon Lee, Professor Won Do Heo from Department of Biological Sciences>
A KAIST research team led by Professor Won Do Heo (Department of Biological Sciences) has developed an optogenetic platform, RELISR (REversible LIght-induced Store and Release), that enables precise spatiotemporal control over the storage and release of proteins and mRNAs in living cells and animals.
Traditional optogenetic condensate systems have been limited by their reliance on non-specific multivalent interactions, which can lead to unintended sequestration or release of endogenous molecules. RELISR overcomes these limitations by employing highly specific protein–protein (nanobody–antigen) and protein–RNA (MCP–MS2) interactions, enabling the selective and reversible compartmentalization of target proteins or mRNAs within engineered, membrane-less condensates.
In the dark, RELISR stably sequesters target molecules within condensates, physically isolating them from the cellular environment. Upon blue light stimulation, the condensates rapidly dissolve, releasing the stored proteins or mRNAs, which immediately regain their cellular functions or translational competency. This allows for reversible and rapid modulation of molecular activities in response to optical cues.
< Figure 1. Overview of the Artificial Condensate System (RELISR). The artificial condensate system, RELISR, includes "Protein-RELISR" for storing proteins and "mRNA-RELISR" for storing mRNA. These artificial condensates can be disassembled by blue light irradiation and reassembled in a dark state>
The research team demonstrated that RELISR enables temporal and spatial regulation of protein activity and mRNA translation in various cell types, including cultured neurons and mouse liver tissue. Comparative studies showed that RELISR provides more robust and reversible control of translation than previous systems based on spatial translocation.
While previous optogenetic systems such as LARIAT (Lee et al., Nature Methods, 2014) and mRNA-LARIAT (Kim et al., Nat. Cell Biol., 2019) enabled the selective sequestration of proteins or mRNAs into membrane-less condensates in response to light, they were primarily limited to the trapping phase. The RELISR platform introduced in this study establishes a new paradigm by enabling both the targeted storage of proteins and mRNAs and their rapid, light-triggered release. This approach allows researchers to not only confine molecular function on demand, but also to restore activity with precise temporal control.
< Figure 2. Cell shape change using the artificial condensate system (RELISR). A target protein, Vav2, which contributes to cell shape, was stored within the artificial condensate and then released after light irradiation. This release activated the target protein Vav2, causing a change in cell shape. It was confirmed that the storage, release, and activation of various proteins were effectively achieved>
Professor Heo stated, “RELISR is a versatile optogenetic tool that enables the precise control of protein and mRNA function at defined times and locations in living systems. We anticipate this platform will be broadly applicable for studies of cell signaling, neural circuits, and therapeutic development. Furthermore, the combination of RELISR with genome editing or tissue-targeted delivery could further expand its utility for molecular medicine.”
< Figure 3. Expression of a target mRNA using the artificial condensate system (RELISR) in mice. The genetic material for the artificial condensate system, RELISR, was injected into a living mouse. Using this system, a target mRNA was stored within the mouse's liver. Upon light irradiation, the mRNA was released, which induced the translation of a luminescent protein>
This research was conducted by first author Dr. Chaeyeon Lee, under the supervision of Professor Heo, with contributions from Dr. Daseuli Yu (co-corresponding author) and Professor YongKeun Park (co-corresponding author, Department of Physics), whose group performed quantitative imaging analyses of biophysical changes induced by RELISR in cells.
The findings were published in Nature Communications (July 7, 2025; DOI: 10.1038/s41467-025-61322-y). This work was supported by the Samsung Future Technology Foundation and the National Research Foundation of Korea.
2025.07.23 View 239
KAIST Team Develops Optogenetic Platform for Spatiotemporal Control of Protein and mRNA Storage and Release
<Dr. Chaeyeon Lee, Professor Won Do Heo from Department of Biological Sciences>
A KAIST research team led by Professor Won Do Heo (Department of Biological Sciences) has developed an optogenetic platform, RELISR (REversible LIght-induced Store and Release), that enables precise spatiotemporal control over the storage and release of proteins and mRNAs in living cells and animals.
Traditional optogenetic condensate systems have been limited by their reliance on non-specific multivalent interactions, which can lead to unintended sequestration or release of endogenous molecules. RELISR overcomes these limitations by employing highly specific protein–protein (nanobody–antigen) and protein–RNA (MCP–MS2) interactions, enabling the selective and reversible compartmentalization of target proteins or mRNAs within engineered, membrane-less condensates.
In the dark, RELISR stably sequesters target molecules within condensates, physically isolating them from the cellular environment. Upon blue light stimulation, the condensates rapidly dissolve, releasing the stored proteins or mRNAs, which immediately regain their cellular functions or translational competency. This allows for reversible and rapid modulation of molecular activities in response to optical cues.
< Figure 1. Overview of the Artificial Condensate System (RELISR). The artificial condensate system, RELISR, includes "Protein-RELISR" for storing proteins and "mRNA-RELISR" for storing mRNA. These artificial condensates can be disassembled by blue light irradiation and reassembled in a dark state>
The research team demonstrated that RELISR enables temporal and spatial regulation of protein activity and mRNA translation in various cell types, including cultured neurons and mouse liver tissue. Comparative studies showed that RELISR provides more robust and reversible control of translation than previous systems based on spatial translocation.
While previous optogenetic systems such as LARIAT (Lee et al., Nature Methods, 2014) and mRNA-LARIAT (Kim et al., Nat. Cell Biol., 2019) enabled the selective sequestration of proteins or mRNAs into membrane-less condensates in response to light, they were primarily limited to the trapping phase. The RELISR platform introduced in this study establishes a new paradigm by enabling both the targeted storage of proteins and mRNAs and their rapid, light-triggered release. This approach allows researchers to not only confine molecular function on demand, but also to restore activity with precise temporal control.
< Figure 2. Cell shape change using the artificial condensate system (RELISR). A target protein, Vav2, which contributes to cell shape, was stored within the artificial condensate and then released after light irradiation. This release activated the target protein Vav2, causing a change in cell shape. It was confirmed that the storage, release, and activation of various proteins were effectively achieved>
Professor Heo stated, “RELISR is a versatile optogenetic tool that enables the precise control of protein and mRNA function at defined times and locations in living systems. We anticipate this platform will be broadly applicable for studies of cell signaling, neural circuits, and therapeutic development. Furthermore, the combination of RELISR with genome editing or tissue-targeted delivery could further expand its utility for molecular medicine.”
< Figure 3. Expression of a target mRNA using the artificial condensate system (RELISR) in mice. The genetic material for the artificial condensate system, RELISR, was injected into a living mouse. Using this system, a target mRNA was stored within the mouse's liver. Upon light irradiation, the mRNA was released, which induced the translation of a luminescent protein>
This research was conducted by first author Dr. Chaeyeon Lee, under the supervision of Professor Heo, with contributions from Dr. Daseuli Yu (co-corresponding author) and Professor YongKeun Park (co-corresponding author, Department of Physics), whose group performed quantitative imaging analyses of biophysical changes induced by RELISR in cells.
The findings were published in Nature Communications (July 7, 2025; DOI: 10.1038/s41467-025-61322-y). This work was supported by the Samsung Future Technology Foundation and the National Research Foundation of Korea.
2025.07.23 View 239 -
 Why Do Plants Attack Themselves? The Secret of Genetic Conflict Revealed
<Professor Ji-Joon Song of the KAIST Department of Biological Sciences>
Plants, with their unique immune systems, sometimes launch 'autoimmune responses' by mistakenly identifying their own protein structures as pathogens. In particular, 'hybrid necrosis,' a phenomenon where descendant plants fail to grow healthily and perish after cross-breeding different varieties, has long been a difficult challenge for botanists and agricultural researchers. In response, an international research team has successfully elucidated the mechanism inducing plant autoimmune responses and proposed a novel strategy for cultivar improvement that can predict and avoid these reactions.
Professor Ji-Joon Song's research team at KAIST, in collaboration with teams from the National University of Singapore (NUS) and the University of Oxford, announced on the 21st of July that they have elucidated the structure and function of the 'DM3' protein complex, which triggers plant autoimmune responses, using cryo-electron microscopy (Cryo-EM) technology.
This research is drawing attention because it identifies defects in protein structure as the cause of hybrid necrosis, which occurs due to an abnormal reaction of immune receptors during cross-breeding between plant hybrids.
This protein (DM3) is originally an enzyme involved in the plant's immune response, but problems arise when the structure of the DM3 protein is damaged in a specific protein combination called 'DANGEROUS MIX (DM)'.
Notably, one variant of DM3, the 'DM3Col-0' variant, forms a stable complex with six proteins and is recognized as normal, thus not triggering an immune response. In contrast, another 'DM3Hh-0' variant has improper binding between its six proteins, causing the plant to recognize it as an 'abnormal state' and trigger an immune alarm, leading to autoimmunity.
The research team visualized this structure using atomic-resolution cryo-electron microscopy (Cryo-EM) and revealed that the immune-inducing ability is not due to the enzymatic function of the DM3 protein, but rather to 'differences in protein binding affinity.'
<Figure 1. Mechanism of Plant Autoimmunity Triggered by the Collapse of the DM3 Protein Complex>
This demonstrates that plants can initiate an immune response by recognizing not only 'external pathogens' but also 'internal protein structures' when they undergo abnormal changes, treating them as if they were pathogens.
The study shows how sensitively the plant immune system changes and triggers autoimmune responses when genes are mixed and protein structures change during the cross-breeding of different plant varieties. It significantly advanced the understanding of genetic incompatibility that can occur during natural cross-breeding and cultivar improvement processes.
Dr. Gijeong Kim, the co-first author, stated, "Through international research collaboration, we presented a new perspective on understanding the plant immune system by leveraging the autoimmune phenomenon, completing a high-quality study that encompasses structural biochemistry, genetics, and cell biological experiments."
Professor Ji-Joon Song of the KAIST Department of Biological Sciences, who led the research, said, "The fact that the immune system can detect not only external pathogens but also structural abnormalities in its own proteins will set a new standard for plant biotechnology and crop breeding strategies. Cryo-electron microscopy-based structural analysis will be an important tool for understanding the essence of gene interactions."
This research, with Professor Ji-Joon Song and Professor Eunyoung Chae of the University of Oxford as co-corresponding authors, Dr. Gijeong Kim (currently a postdoctoral researcher at the University of Zurich) and Dr. Wei-Lin Wan of the National University of Singapore as co-first authors, and Ph.D candidate Nayun Kim, as the second author, was published on July 17th in Molecular Cell, a sister journal of the international academic journal Cell.
This research was supported by the KAIST Grand Challenge 30 project.
Article Title: Structural determinants of DANGEROUS MIX 3, an alpha/beta hydrolase that triggers NLR-mediated genetic incompatibility in plants DOI: https://doi.org/10.1016/j.molcel.2025.06.021
2025.07.21 View 390
Why Do Plants Attack Themselves? The Secret of Genetic Conflict Revealed
<Professor Ji-Joon Song of the KAIST Department of Biological Sciences>
Plants, with their unique immune systems, sometimes launch 'autoimmune responses' by mistakenly identifying their own protein structures as pathogens. In particular, 'hybrid necrosis,' a phenomenon where descendant plants fail to grow healthily and perish after cross-breeding different varieties, has long been a difficult challenge for botanists and agricultural researchers. In response, an international research team has successfully elucidated the mechanism inducing plant autoimmune responses and proposed a novel strategy for cultivar improvement that can predict and avoid these reactions.
Professor Ji-Joon Song's research team at KAIST, in collaboration with teams from the National University of Singapore (NUS) and the University of Oxford, announced on the 21st of July that they have elucidated the structure and function of the 'DM3' protein complex, which triggers plant autoimmune responses, using cryo-electron microscopy (Cryo-EM) technology.
This research is drawing attention because it identifies defects in protein structure as the cause of hybrid necrosis, which occurs due to an abnormal reaction of immune receptors during cross-breeding between plant hybrids.
This protein (DM3) is originally an enzyme involved in the plant's immune response, but problems arise when the structure of the DM3 protein is damaged in a specific protein combination called 'DANGEROUS MIX (DM)'.
Notably, one variant of DM3, the 'DM3Col-0' variant, forms a stable complex with six proteins and is recognized as normal, thus not triggering an immune response. In contrast, another 'DM3Hh-0' variant has improper binding between its six proteins, causing the plant to recognize it as an 'abnormal state' and trigger an immune alarm, leading to autoimmunity.
The research team visualized this structure using atomic-resolution cryo-electron microscopy (Cryo-EM) and revealed that the immune-inducing ability is not due to the enzymatic function of the DM3 protein, but rather to 'differences in protein binding affinity.'
<Figure 1. Mechanism of Plant Autoimmunity Triggered by the Collapse of the DM3 Protein Complex>
This demonstrates that plants can initiate an immune response by recognizing not only 'external pathogens' but also 'internal protein structures' when they undergo abnormal changes, treating them as if they were pathogens.
The study shows how sensitively the plant immune system changes and triggers autoimmune responses when genes are mixed and protein structures change during the cross-breeding of different plant varieties. It significantly advanced the understanding of genetic incompatibility that can occur during natural cross-breeding and cultivar improvement processes.
Dr. Gijeong Kim, the co-first author, stated, "Through international research collaboration, we presented a new perspective on understanding the plant immune system by leveraging the autoimmune phenomenon, completing a high-quality study that encompasses structural biochemistry, genetics, and cell biological experiments."
Professor Ji-Joon Song of the KAIST Department of Biological Sciences, who led the research, said, "The fact that the immune system can detect not only external pathogens but also structural abnormalities in its own proteins will set a new standard for plant biotechnology and crop breeding strategies. Cryo-electron microscopy-based structural analysis will be an important tool for understanding the essence of gene interactions."
This research, with Professor Ji-Joon Song and Professor Eunyoung Chae of the University of Oxford as co-corresponding authors, Dr. Gijeong Kim (currently a postdoctoral researcher at the University of Zurich) and Dr. Wei-Lin Wan of the National University of Singapore as co-first authors, and Ph.D candidate Nayun Kim, as the second author, was published on July 17th in Molecular Cell, a sister journal of the international academic journal Cell.
This research was supported by the KAIST Grand Challenge 30 project.
Article Title: Structural determinants of DANGEROUS MIX 3, an alpha/beta hydrolase that triggers NLR-mediated genetic incompatibility in plants DOI: https://doi.org/10.1016/j.molcel.2025.06.021
2025.07.21 View 390 -
 KAIST's Lim Mi-hee wins Korea's L'Oréal-UNESCO Women in Science Award
Lim Mi-hee, a professor at the Korea Advanced Institute of Science and Technology (KAIST) Department of Chemistry, received the Academic Promotion Award at the 24th Korean L'Oréal-UNESCO Women in Science Awards ceremony.
L'Oréal Korea, the Korean National Commission for UNESCO, and the Women’s Bioscience Forum held the 24th Korean L'Oréal-UNESCO Women in Science Awards ceremony on the 16th and noted that Lim Mi-hee was selected for this year’s Academic Promotion Award.
Professor Lim was recognized for her research on the causes of Alzheimer's disease at the molecular level and her efforts in the discovery of intracellular proteins that promote the toxicity of Alzheimer’s-inducing factors. Professor Lim is a full member of the Korean Academy of Science and Technology (KAST) and has received several awards including the Hanseong Science Award, this year's Women in Science and Technology Award, and the RIGAKU-ACCC Award (Asia's top woman scientist).
The fellowship section, awarded to four emerging women scientists, includes Kang Mi-kyung, an assistant professor at Korea University’s Department of Health and Environmental Sciences; Jeon Ji-hye, an assistant professor at Gyeongsang National University’s Department of Life Sciences; Jo Yu-na, a research professor at Pusan National University’s College of Medicine; and Lee Jeong-hyun, an assistant professor at Kongju National University’s Department of Environmental Education.
The recipients of the Academic Promotion Award and fellowships will receive a certificate and a trophy, along with research funding of 30 million won and 7 million won, respectively.
Samuel du Retail, the representative of L'Oréal Korea, said, “The L'Oréal Group continues to support the empowerment of women scientists and the improvement of research environments worldwide under the philosophy that 'the world needs science, and science needs women.' We will actively support more female talents to shine at the center of scientific and technological advancement in the future.”
2025.07.18 View 266
KAIST's Lim Mi-hee wins Korea's L'Oréal-UNESCO Women in Science Award
Lim Mi-hee, a professor at the Korea Advanced Institute of Science and Technology (KAIST) Department of Chemistry, received the Academic Promotion Award at the 24th Korean L'Oréal-UNESCO Women in Science Awards ceremony.
L'Oréal Korea, the Korean National Commission for UNESCO, and the Women’s Bioscience Forum held the 24th Korean L'Oréal-UNESCO Women in Science Awards ceremony on the 16th and noted that Lim Mi-hee was selected for this year’s Academic Promotion Award.
Professor Lim was recognized for her research on the causes of Alzheimer's disease at the molecular level and her efforts in the discovery of intracellular proteins that promote the toxicity of Alzheimer’s-inducing factors. Professor Lim is a full member of the Korean Academy of Science and Technology (KAST) and has received several awards including the Hanseong Science Award, this year's Women in Science and Technology Award, and the RIGAKU-ACCC Award (Asia's top woman scientist).
The fellowship section, awarded to four emerging women scientists, includes Kang Mi-kyung, an assistant professor at Korea University’s Department of Health and Environmental Sciences; Jeon Ji-hye, an assistant professor at Gyeongsang National University’s Department of Life Sciences; Jo Yu-na, a research professor at Pusan National University’s College of Medicine; and Lee Jeong-hyun, an assistant professor at Kongju National University’s Department of Environmental Education.
The recipients of the Academic Promotion Award and fellowships will receive a certificate and a trophy, along with research funding of 30 million won and 7 million won, respectively.
Samuel du Retail, the representative of L'Oréal Korea, said, “The L'Oréal Group continues to support the empowerment of women scientists and the improvement of research environments worldwide under the philosophy that 'the world needs science, and science needs women.' We will actively support more female talents to shine at the center of scientific and technological advancement in the future.”
2025.07.18 View 266 -
 KAIST Develops Robots That React to Danger Like Humans
<(From left) Ph.D candidate See-On Park, Professor Jongwon Lee, and Professor Shinhyun Choi>
In the midst of the co-development of artificial intelligence and robotic advancements, developing technologies that enable robots to efficiently perceive and respond to their surroundings like humans has become a crucial task. In this context, Korean researchers are gaining attention for newly implementing an artificial sensory nervous system that mimics the sensory nervous system of living organisms without the need for separate complex software or circuitry. This breakthrough technology is expected to be applied in fields such as in ultra-small robots and robotic prosthetics, where intelligent and energy-efficient responses to external stimuli are essential.
KAIST (President Kwang Hyung Lee) announced on July15th that a joint research team led by Endowed Chair Professor Shinhyun Choi of the School of Electrical Engineering at KAIST and Professor Jongwon Lee of the Department of Semiconductor Convergence at Chungnam National University (President Jung Kyum Kim) developed a next-generation neuromorphic semiconductor-based artificial sensory nervous system. This system mimics the functions of a living organism's sensory nervous system, and enables a new type of robotic system that can efficiently responds to external stimuli.
In nature, animals — including humans — ignore safe or familiar stimuli and selectively react sensitively to important or dangerous ones. This selective response helps prevent unnecessary energy consumption while maintaining rapid awareness of critical signals. For instance, the sound of an air conditioner or the feel of clothing against the skin soon become familiar and are disregarded. However, if someone calls your name or a sharp object touches your skin, a rapid focus and response occur. These behaviors are regulated by the 'habituation' and 'sensitization' functions in the sensory nervous system. Attempts have been consistently made to apply these sensory nervous system functions of living organisms in order to create robots that efficiently respond to external environments like humans.
However, implementing complex neural characteristics such as habituation and sensitization in robots has faced difficulties in miniaturization and energy efficiency due to the need for separate software or complex circuitry. In particular, there have been attempts to utilize memristors, a neuromorphic semiconductor. A memristor is a next-generation electrical device, which has been widely utilized as an artificial synapse due to its ability to store analog value in the form of device resistance. However, existing memristors had limitations in mimicking the complex characteristics of the nervous system because they only allowed simple monotonic changes in conductivity.
To overcome these limitations, the research team developed a new memristor capable of reproducing complex neural response patterns such as habituation and sensitization within a single device. By introducing additional layer inside the memristor that alter conductivity in opposite directions, the device can more realistically emulate the dynamic synaptic behaviors of a real nervous system — for example, decreasing its response to repeated safe stimuli but quickly regaining sensitivity when a danger signal is detected.
<New memristor mimicking functions of sensory nervous system such as habituation/sensitization>
Using this new memristor, the research team built an artificial sensory nervous system capable of recognizing touch and pain, an applied it to a robotic hand to test its performance. When safe tactile stimuli were repeatedly applied, the robot hand, which initially reacted sensitively to unfamiliar tactile stimuli, gradually showed habituation characteristics by ignoring the stimuli. Later, when stimuli were applied along with an electric shock, it recognized this as a danger signal and showed sensitization characteristics by reacting sensitively again. Through this, it was experimentally proven that robots can efficiently respond to stimuli like humans without separate complex software or processors, verifying the possibility of developing energy-efficient neuro-inspired robots.
<Robot arm with memristor-based artificial sensory nervous system>
See-On Park, researcher at KAIST, stated, "By mimicking the human sensory nervous system with next-generation semiconductors, we have opened up the possibility of implementing a new concept of robots that are smarter and more energy-efficient in responding to external environments." He added, "This technology is expected to be utilized in various fusion fields of next-generation semiconductors and robotics, such as ultra-small robots, military robots, and medical robots like robotic prosthetics".
This research was published online on July 1st in the international journal 'Nature Communications,' with Ph.D candidate See-On Park as the first author.
Paper Title: Experimental demonstration of third-order memristor-based artificial sensory nervous system for neuro-inspired robotics
DOI: https://doi.org/10.1038/s41467-025-60818-x
This research was supported by the Korea National Research Foundation's Next-Generation Intelligent Semiconductor Technology Development Project, the Mid-Career Researcher Program, the PIM Artificial Intelligence Semiconductor Core Technology Development Project, the Excellent New Researcher Program, and the Nano Convergence Technology Division, National Nanofab Center's (NNFC) Nano-Medical Device Project.
2025.07.16 View 638
KAIST Develops Robots That React to Danger Like Humans
<(From left) Ph.D candidate See-On Park, Professor Jongwon Lee, and Professor Shinhyun Choi>
In the midst of the co-development of artificial intelligence and robotic advancements, developing technologies that enable robots to efficiently perceive and respond to their surroundings like humans has become a crucial task. In this context, Korean researchers are gaining attention for newly implementing an artificial sensory nervous system that mimics the sensory nervous system of living organisms without the need for separate complex software or circuitry. This breakthrough technology is expected to be applied in fields such as in ultra-small robots and robotic prosthetics, where intelligent and energy-efficient responses to external stimuli are essential.
KAIST (President Kwang Hyung Lee) announced on July15th that a joint research team led by Endowed Chair Professor Shinhyun Choi of the School of Electrical Engineering at KAIST and Professor Jongwon Lee of the Department of Semiconductor Convergence at Chungnam National University (President Jung Kyum Kim) developed a next-generation neuromorphic semiconductor-based artificial sensory nervous system. This system mimics the functions of a living organism's sensory nervous system, and enables a new type of robotic system that can efficiently responds to external stimuli.
In nature, animals — including humans — ignore safe or familiar stimuli and selectively react sensitively to important or dangerous ones. This selective response helps prevent unnecessary energy consumption while maintaining rapid awareness of critical signals. For instance, the sound of an air conditioner or the feel of clothing against the skin soon become familiar and are disregarded. However, if someone calls your name or a sharp object touches your skin, a rapid focus and response occur. These behaviors are regulated by the 'habituation' and 'sensitization' functions in the sensory nervous system. Attempts have been consistently made to apply these sensory nervous system functions of living organisms in order to create robots that efficiently respond to external environments like humans.
However, implementing complex neural characteristics such as habituation and sensitization in robots has faced difficulties in miniaturization and energy efficiency due to the need for separate software or complex circuitry. In particular, there have been attempts to utilize memristors, a neuromorphic semiconductor. A memristor is a next-generation electrical device, which has been widely utilized as an artificial synapse due to its ability to store analog value in the form of device resistance. However, existing memristors had limitations in mimicking the complex characteristics of the nervous system because they only allowed simple monotonic changes in conductivity.
To overcome these limitations, the research team developed a new memristor capable of reproducing complex neural response patterns such as habituation and sensitization within a single device. By introducing additional layer inside the memristor that alter conductivity in opposite directions, the device can more realistically emulate the dynamic synaptic behaviors of a real nervous system — for example, decreasing its response to repeated safe stimuli but quickly regaining sensitivity when a danger signal is detected.
<New memristor mimicking functions of sensory nervous system such as habituation/sensitization>
Using this new memristor, the research team built an artificial sensory nervous system capable of recognizing touch and pain, an applied it to a robotic hand to test its performance. When safe tactile stimuli were repeatedly applied, the robot hand, which initially reacted sensitively to unfamiliar tactile stimuli, gradually showed habituation characteristics by ignoring the stimuli. Later, when stimuli were applied along with an electric shock, it recognized this as a danger signal and showed sensitization characteristics by reacting sensitively again. Through this, it was experimentally proven that robots can efficiently respond to stimuli like humans without separate complex software or processors, verifying the possibility of developing energy-efficient neuro-inspired robots.
<Robot arm with memristor-based artificial sensory nervous system>
See-On Park, researcher at KAIST, stated, "By mimicking the human sensory nervous system with next-generation semiconductors, we have opened up the possibility of implementing a new concept of robots that are smarter and more energy-efficient in responding to external environments." He added, "This technology is expected to be utilized in various fusion fields of next-generation semiconductors and robotics, such as ultra-small robots, military robots, and medical robots like robotic prosthetics".
This research was published online on July 1st in the international journal 'Nature Communications,' with Ph.D candidate See-On Park as the first author.
Paper Title: Experimental demonstration of third-order memristor-based artificial sensory nervous system for neuro-inspired robotics
DOI: https://doi.org/10.1038/s41467-025-60818-x
This research was supported by the Korea National Research Foundation's Next-Generation Intelligent Semiconductor Technology Development Project, the Mid-Career Researcher Program, the PIM Artificial Intelligence Semiconductor Core Technology Development Project, the Excellent New Researcher Program, and the Nano Convergence Technology Division, National Nanofab Center's (NNFC) Nano-Medical Device Project.
2025.07.16 View 638 -
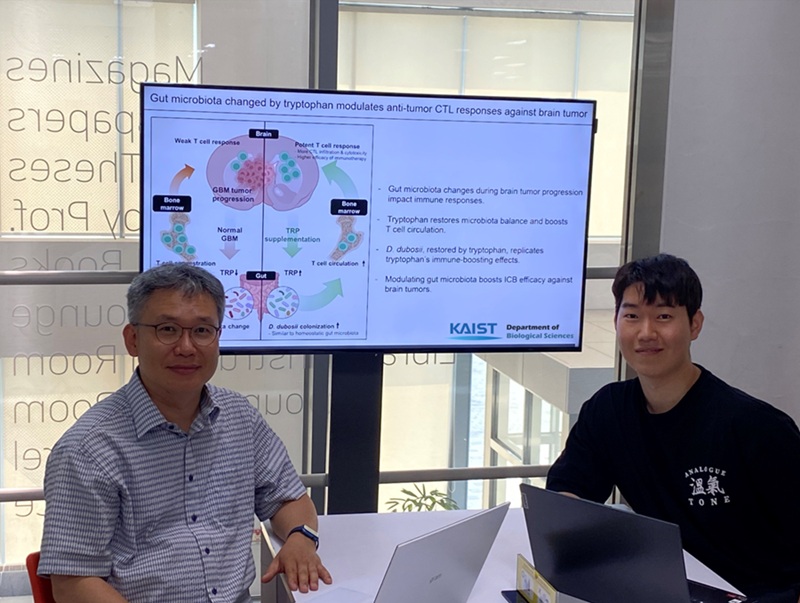 KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 1260
KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 1260 -
 KAIST Invites World-Renowned Scholars, Elevating Global Competitiveness
< Photo 1. (From left) Professor John Rogers, Professor Gregg Rothermel, Dr. Sang H. Choi >
KAIST announced on June 27th that it has appointed three world-renowned scholars, including Professor John A. Rogers of Northwestern University, USA, as Invited Distinguished Professors in key departments such as Materials Science and Engineering.
Professor John A. Rogers (Northwestern University, USA) will be working with the Department of Materials Science and Engineering from July 2025 to June 2028 with Professor Gregg Rothermel (North Carolina State University, USA) working with the School of Computing from August 2025 to July 2026, and Dr. Sang H. Choi (NASA Langley Research Center, USA) with the Department of Aerospace Engineering from May 2025 to April 2028.
Professor John A. Rogers, a person of global authority in the field of bio-integrated electronics, has been leading advanced convergence technologies such as flexible electronics, smart skin, and implantable sensors. His significant impact on academia and industry is evident through over 900 papers published in top-tier academic journals like Science, Nature, and Cell, and he comes in an H-index of 240*. His research group, the Rogers Research Group at Northwestern University, focuses on "Science that brings Solutions to Society," encompassing areas such as bio-integrated microsystems and unconventional nanofabrication techniques. He is the founding Director of the Querrey-Simpson Institute of Bioelectronics at Northwestern University.
* H-index 240: An H-index is a measurement used to assess the research productivity and impact of an individual authors. H-index 240 means that 240 or more papers have been cited at least 240 times each, indicating a significant impact and the presumable status as a world-class scholar.
The Department of Materials Science and Engineering plans to further enhance its research capabilities in next-generation bio-implantable materials and wearable devices and boost its global competitiveness through the invitation of Professor Rogers. In particular, it aims to create strong research synergies by linking with the development of bio-convergence interface materials, a core task of the Leading Research Center (ERC, total research budget of 13.5 billion KRW over 7 years) led by Professor Kun-Jae Lee.
Professor Gregg Rothermel, a world-renowned scholar in software engineering, was ranked second among the top 50 global researchers by Communications of the ACM. For over 30 years, he has conducted practical research to improve software reliability and quality. He has achieved influential research outcomes through collaborations with global companies such as Boeing, Microsoft, and Lockheed Martin. Dr. Rothermel's research at North Carolina State University focuses on software engineering and program analysis, with significant contributions through initiatives like the ESQuaReD Laboratory and the Software-Artifact Infrastructure Repository (SIR).
The School of Computing plans to strengthen its research capabilities in software engineering and conduct collaborative research on software design and testing to enhance the reliability and safety of AI-based software systems through the invitation of Professor Gregg Rothermel. In particular, he is expected to participate in the Big Data Edge-Cloud Service Research Center (ITRC, total research budget of 6.7 billion KRW over 8 years) led by Professor In-Young Ko of the School of Computing, and the Research on Improving Complex Mobility Safety (SafetyOps, Digital Columbus Project, total research budget of 3.5 billion KRW over 8 years), contributing to resolving uncertainties in machine learning-based AI software and advancing technology.
Dr. Sang H. Choi, a global expert in space exploration and energy harvesting, has worked at NASA Langley Research Center for over 40 years, authoring over 200 papers and reports, holding 45 patents, and receiving 71 awards from NASA. In 2022, he was inducted into the 'Inventors Hall of Fame' as part of NASA's Technology Transfer Program. This is a rare honor, recognizing researchers who have contributed to the private sector dissemination of space exploration technology, with only 35 individuals worldwide selected to date. Dr. Choi's extensive work at NASA includes research on advanced electronic and energetic materials, satellite sensors, and various nano-technologies.
Dr. Choi plans to collaborate with Associate Professor Hyun-Jung Kim (former NASA Research Scientist, 2009-2024), who joined the Department of Aerospace Engineering in September of 2024, to lead the development of core technologies for lunar exploration (energy sources, sensing, in-situ resource utilization ISRU).
KAIST President Kwang Hyung Lee stated, "It is very meaningful to be able to invite these world-class scholars. Through these appointments, KAIST will further strengthen its global competitiveness in research in the fields of advanced convergence technology such as bio-convergence electronics, AI software engineering, and space exploration, securing our position as the leader of global innovations."
2025.06.27 View 2095
KAIST Invites World-Renowned Scholars, Elevating Global Competitiveness
< Photo 1. (From left) Professor John Rogers, Professor Gregg Rothermel, Dr. Sang H. Choi >
KAIST announced on June 27th that it has appointed three world-renowned scholars, including Professor John A. Rogers of Northwestern University, USA, as Invited Distinguished Professors in key departments such as Materials Science and Engineering.
Professor John A. Rogers (Northwestern University, USA) will be working with the Department of Materials Science and Engineering from July 2025 to June 2028 with Professor Gregg Rothermel (North Carolina State University, USA) working with the School of Computing from August 2025 to July 2026, and Dr. Sang H. Choi (NASA Langley Research Center, USA) with the Department of Aerospace Engineering from May 2025 to April 2028.
Professor John A. Rogers, a person of global authority in the field of bio-integrated electronics, has been leading advanced convergence technologies such as flexible electronics, smart skin, and implantable sensors. His significant impact on academia and industry is evident through over 900 papers published in top-tier academic journals like Science, Nature, and Cell, and he comes in an H-index of 240*. His research group, the Rogers Research Group at Northwestern University, focuses on "Science that brings Solutions to Society," encompassing areas such as bio-integrated microsystems and unconventional nanofabrication techniques. He is the founding Director of the Querrey-Simpson Institute of Bioelectronics at Northwestern University.
* H-index 240: An H-index is a measurement used to assess the research productivity and impact of an individual authors. H-index 240 means that 240 or more papers have been cited at least 240 times each, indicating a significant impact and the presumable status as a world-class scholar.
The Department of Materials Science and Engineering plans to further enhance its research capabilities in next-generation bio-implantable materials and wearable devices and boost its global competitiveness through the invitation of Professor Rogers. In particular, it aims to create strong research synergies by linking with the development of bio-convergence interface materials, a core task of the Leading Research Center (ERC, total research budget of 13.5 billion KRW over 7 years) led by Professor Kun-Jae Lee.
Professor Gregg Rothermel, a world-renowned scholar in software engineering, was ranked second among the top 50 global researchers by Communications of the ACM. For over 30 years, he has conducted practical research to improve software reliability and quality. He has achieved influential research outcomes through collaborations with global companies such as Boeing, Microsoft, and Lockheed Martin. Dr. Rothermel's research at North Carolina State University focuses on software engineering and program analysis, with significant contributions through initiatives like the ESQuaReD Laboratory and the Software-Artifact Infrastructure Repository (SIR).
The School of Computing plans to strengthen its research capabilities in software engineering and conduct collaborative research on software design and testing to enhance the reliability and safety of AI-based software systems through the invitation of Professor Gregg Rothermel. In particular, he is expected to participate in the Big Data Edge-Cloud Service Research Center (ITRC, total research budget of 6.7 billion KRW over 8 years) led by Professor In-Young Ko of the School of Computing, and the Research on Improving Complex Mobility Safety (SafetyOps, Digital Columbus Project, total research budget of 3.5 billion KRW over 8 years), contributing to resolving uncertainties in machine learning-based AI software and advancing technology.
Dr. Sang H. Choi, a global expert in space exploration and energy harvesting, has worked at NASA Langley Research Center for over 40 years, authoring over 200 papers and reports, holding 45 patents, and receiving 71 awards from NASA. In 2022, he was inducted into the 'Inventors Hall of Fame' as part of NASA's Technology Transfer Program. This is a rare honor, recognizing researchers who have contributed to the private sector dissemination of space exploration technology, with only 35 individuals worldwide selected to date. Dr. Choi's extensive work at NASA includes research on advanced electronic and energetic materials, satellite sensors, and various nano-technologies.
Dr. Choi plans to collaborate with Associate Professor Hyun-Jung Kim (former NASA Research Scientist, 2009-2024), who joined the Department of Aerospace Engineering in September of 2024, to lead the development of core technologies for lunar exploration (energy sources, sensing, in-situ resource utilization ISRU).
KAIST President Kwang Hyung Lee stated, "It is very meaningful to be able to invite these world-class scholars. Through these appointments, KAIST will further strengthen its global competitiveness in research in the fields of advanced convergence technology such as bio-convergence electronics, AI software engineering, and space exploration, securing our position as the leader of global innovations."
2025.06.27 View 2095 -
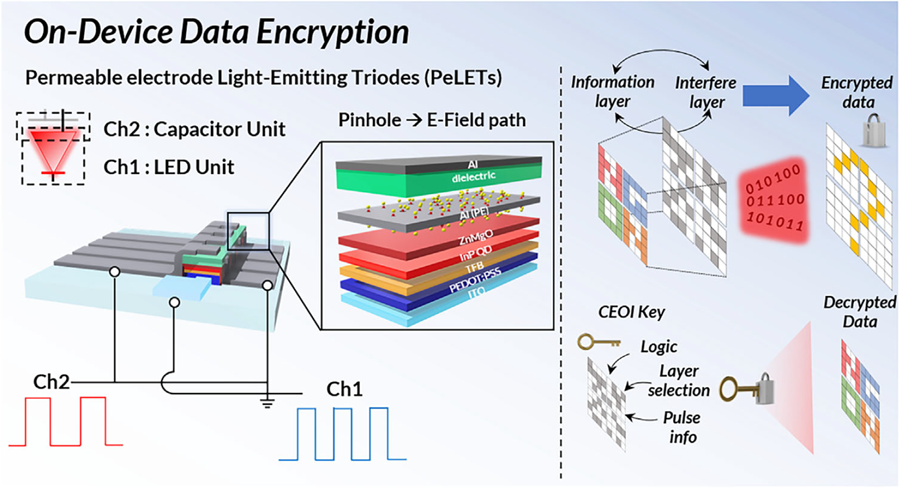 KAIST's Li-Fi - Achieves 100 Times Faster Speed and Enhanced Security of Wi-Fi
- KAIST-KRISS Develop 'On-Device Encryption Optical Transmitter' Based on Eco-Friendly Quantum Dots
- New Li-Fi Platform Technology Achieves High Performance with 17.4% Device Efficiency and 29,000 nit Brightness, Simultaneously Improving Transmission Speed and Security
- Presents New Methodology for High-Speed and Encrypted Communication Through Single-Device-Based Dual-Channel Optical Modulation
< Photo 1. (Front row from left) Seungmin Shin, First Author; Professor Himchan Cho; (Back row from left) Hyungdoh Lee, Seungwoo Lee, Wonbeom Lee; (Top left) Dr. Kyung-geun Lim >
Li-Fi (Light Fidelity) is a wireless communication technology that utilizes the visible light spectrum (400-800 THz), similar to LED light, offering speeds up to 100 times faster than existing Wi-Fi (up to 224 Gbps). While it has fewer limitations in available frequency allocation and less radio interference, it is relatively vulnerable to security breaches as anyone can access it. Korean researchers have now proposed a new Li-Fi platform that overcomes the limitations of conventional optical communication devices and can simultaneously enhance both transmission speed and security.
KAIST (President Kwang Hyung Lee) announced on the 24th that Professor Himchan Cho's research team from the Department of Materials Science and Engineering, in collaboration with Dr. Kyung-geun Lim of the Korea Research Institute of Standards and Science (KRISS, President Ho-Seong Lee) under the National Research Council of Science & Technology (NST, Chairman Young-Sik Kim), has developed 'on-device encryption optical communication device' technology for the utilization of 'Li-Fi,' which is attracting attention as a next-generation ultra-high-speed data communication.
Professor Cho's team created high-efficiency light-emitting triode devices using eco-friendly quantum dots (low-toxicity and sustainable materials). The device developed by the research team is a mechanism that generates light using an electric field. Specifically, the electric field is concentrated in 'tiny holes (pinholes) in the permeable electrode' and transmitted beyond the electrode. This device utilizes this principle to simultaneously process two input data streams.
Using this principle, the research team developed a technology called 'on-device encryption optical transmitter.' The core of this technology is that the device itself converts information into light and simultaneously encrypts it. This means that enhanced security data transmission is possible without the need for complex, separate equipment.
External Quantum Efficiency (EQE) is an indicator of how efficiently electricity is converted into light, with a general commercialization standard of about 20%. The newly developed device recorded an EQE of 17.4%, and its luminance was 29,000 nit, significantly exceeding the maximum brightness of a smartphone OLED screen, which is 2,000 nit, demonstrating a brightness more than 10 times higher.
< Figure 1. Schematic diagram of the device structure developed by the research team and encrypted communication >
Furthermore, to more accurately understand how this device converts information into light, the research team used a method called 'transient electroluminescence analysis.' They analyzed the light-emitting characteristics generated by the device when voltage was instantaneously applied for very short durations (hundreds of nanoseconds = billionths of a second). Through this analysis, they investigated the movement of charges within the device at hundreds of nanoseconds, elucidating the operating mechanism of dual-channel optical modulation implemented within a single device.
Professor Himchan Cho of KAIST stated, "This research overcomes the limitations of existing optical communication devices and proposes a new communication platform that can both increase transmission speed and enhance security."
< Photo 2. Professor Himchan Cho, Department of Materials Science and Engineering >
He added, "This technology, which strengthens security without additional equipment and simultaneously enables encryption and transmission, can be widely applied in various fields where security is crucial in the future."
This research, with Seungmin Shin, a Ph.D. candidate at KAIST's Department of Materials Science and Engineering, participating as the first author, and Professor Himchan Cho and Dr. Kyung-geun Lim of KRISS as co-corresponding authors, was published in the international journal 'Advanced Materials' on May 30th and was selected as an inside front cover paper.※ Paper Title: High-Efficiency Quantum Dot Permeable electrode Light-Emitting Triodes for Visible-Light Communications and On-Device Data Encryption※ DOI: https://doi.org/10.1002/adma.202503189
This research was supported by the National Research Foundation of Korea, the National Research Council of Science & Technology (NST), and the Korea Institute for Advancement of Technology.
2025.06.24 View 2449
KAIST's Li-Fi - Achieves 100 Times Faster Speed and Enhanced Security of Wi-Fi
- KAIST-KRISS Develop 'On-Device Encryption Optical Transmitter' Based on Eco-Friendly Quantum Dots
- New Li-Fi Platform Technology Achieves High Performance with 17.4% Device Efficiency and 29,000 nit Brightness, Simultaneously Improving Transmission Speed and Security
- Presents New Methodology for High-Speed and Encrypted Communication Through Single-Device-Based Dual-Channel Optical Modulation
< Photo 1. (Front row from left) Seungmin Shin, First Author; Professor Himchan Cho; (Back row from left) Hyungdoh Lee, Seungwoo Lee, Wonbeom Lee; (Top left) Dr. Kyung-geun Lim >
Li-Fi (Light Fidelity) is a wireless communication technology that utilizes the visible light spectrum (400-800 THz), similar to LED light, offering speeds up to 100 times faster than existing Wi-Fi (up to 224 Gbps). While it has fewer limitations in available frequency allocation and less radio interference, it is relatively vulnerable to security breaches as anyone can access it. Korean researchers have now proposed a new Li-Fi platform that overcomes the limitations of conventional optical communication devices and can simultaneously enhance both transmission speed and security.
KAIST (President Kwang Hyung Lee) announced on the 24th that Professor Himchan Cho's research team from the Department of Materials Science and Engineering, in collaboration with Dr. Kyung-geun Lim of the Korea Research Institute of Standards and Science (KRISS, President Ho-Seong Lee) under the National Research Council of Science & Technology (NST, Chairman Young-Sik Kim), has developed 'on-device encryption optical communication device' technology for the utilization of 'Li-Fi,' which is attracting attention as a next-generation ultra-high-speed data communication.
Professor Cho's team created high-efficiency light-emitting triode devices using eco-friendly quantum dots (low-toxicity and sustainable materials). The device developed by the research team is a mechanism that generates light using an electric field. Specifically, the electric field is concentrated in 'tiny holes (pinholes) in the permeable electrode' and transmitted beyond the electrode. This device utilizes this principle to simultaneously process two input data streams.
Using this principle, the research team developed a technology called 'on-device encryption optical transmitter.' The core of this technology is that the device itself converts information into light and simultaneously encrypts it. This means that enhanced security data transmission is possible without the need for complex, separate equipment.
External Quantum Efficiency (EQE) is an indicator of how efficiently electricity is converted into light, with a general commercialization standard of about 20%. The newly developed device recorded an EQE of 17.4%, and its luminance was 29,000 nit, significantly exceeding the maximum brightness of a smartphone OLED screen, which is 2,000 nit, demonstrating a brightness more than 10 times higher.
< Figure 1. Schematic diagram of the device structure developed by the research team and encrypted communication >
Furthermore, to more accurately understand how this device converts information into light, the research team used a method called 'transient electroluminescence analysis.' They analyzed the light-emitting characteristics generated by the device when voltage was instantaneously applied for very short durations (hundreds of nanoseconds = billionths of a second). Through this analysis, they investigated the movement of charges within the device at hundreds of nanoseconds, elucidating the operating mechanism of dual-channel optical modulation implemented within a single device.
Professor Himchan Cho of KAIST stated, "This research overcomes the limitations of existing optical communication devices and proposes a new communication platform that can both increase transmission speed and enhance security."
< Photo 2. Professor Himchan Cho, Department of Materials Science and Engineering >
He added, "This technology, which strengthens security without additional equipment and simultaneously enables encryption and transmission, can be widely applied in various fields where security is crucial in the future."
This research, with Seungmin Shin, a Ph.D. candidate at KAIST's Department of Materials Science and Engineering, participating as the first author, and Professor Himchan Cho and Dr. Kyung-geun Lim of KRISS as co-corresponding authors, was published in the international journal 'Advanced Materials' on May 30th and was selected as an inside front cover paper.※ Paper Title: High-Efficiency Quantum Dot Permeable electrode Light-Emitting Triodes for Visible-Light Communications and On-Device Data Encryption※ DOI: https://doi.org/10.1002/adma.202503189
This research was supported by the National Research Foundation of Korea, the National Research Council of Science & Technology (NST), and the Korea Institute for Advancement of Technology.
2025.06.24 View 2449 -
 ‘InnoCORE Research Group’ Launched to Lead AI Convergence Innovation
KAIST announced on the 16th of June that it has launched the ‘InnoCORE (Innovation-Core) Research Group,’ which will lead advanced strategic research in AI convergence (AI+S&T), in cooperation with the Ministry of Science and ICT (Minister Yoo Sang-im, hereinafter referred to as MSIT) and DGIST, GIST, and UNIST*. Through this, the group plans to actively recruit up to 200 world-class postdoctoral researchers.
DGIST (Daegu Gyeongbuk Institute of Science & Technology), GIST (Gwangju Institute of Science & Technology), UNIST (Ulsan National Institute of Science and Technology)
The ‘InnoCORE Research Group’ aims to foster core research personnel who will lead innovation in the field of AI convergence, focusing on nurturing and attracting high-level research talent in AI+Science & Technology. This is a strategic response to prevent brain drain of domestic talent and attract excellent overseas talent amidst the accelerating global competition for AI talent.
Through this initiative, our university plans to accelerate AI-based science and technology innovation and disseminate research achievements across industries and the economy by supporting top domestic and international postdoctoral researchers to dedicate themselves to developing AI convergence technologies in an advanced collaborative research environment.
The InnoCORE project for advanced AI+S&T convergence research and global talent attraction is jointly promoted by four science and technology institutes, including KAIST. It is structured around AI core technologies (such as hyper-scale language models, AI semiconductors) and AI convergence technologies (such as bio, manufacturing, energy, and aerospace).
As the leading institution, our university operates the following four research groups:
Hyper-scale Language Model Innovation Research Group: Advancement of LLM technology and research on generative AI, multimodal AI, and ensuring reliability.
AI-based Intelligent Design-Manufacturing Integration Research Group: Establishment of an AI platform for the entire lifecycle of the manufacturing industry and innovation in design and processes.
AI-Innovation Drug Research Group: Securing AI-based drug development technologies across the entire lifecycle and overcoming intractable diseases.
AI-Transformed Aerospace Research Group: AI transformation of aerospace systems throughout their lifecycle and development of new technologies such as autonomous flight and space communication.
< Poster on the InnoCORE Global Jobfair for Recruitment of Postdoctoral Researchers >
In addition, a total of eight research groups are formed to promote global collaborative convergence research, including those led by DGIST, GIST, and UNIST: ▲Bio-Integrated Physical AI, ▲Early Diagnosis of Brain Diseases AI+Nano Convergence, ▲Intelligent Hydrogen Technology Innovation, and ▲AI-Space Solar Power Research Group.
Starting in 2025, the four science and technology institutes, including KAIST, will officially begin recruiting 400 postdoctoral researchers in the AI+S&T fields. Selected postdoctoral researchers will be guaranteed high-level treatment with an annual salary of over 90 million KRW, and additional support through matching with companies and research projects is also planned.
In particular, global recruitment fairs will be held in major US regions to expand the attraction of excellent overseas talent. Local recruitment fairs will be held in Boston (Harvard, MIT), New York (NYU), and Silicon Valley (Stanford) in June, along with promotions through global academic journals such as Nature and Science, and LinkedIn.
KAIST plans to provide multiple mentor programs, global joint research opportunities, and excellent infrastructure (such as supercomputers, semiconductor fabs, and AI research platforms) within the research groups to enable postdoctoral researchers to collaborate with experts from various academic and industrial fields.
President Kwang Hyung Lee emphasized, “Through this InnoCORE project, KAIST will leap forward as a Global Hub for AI+S&T convergence research. Young researchers from around the world will challenge themselves and grow at KAIST, and our country will play a pivotal role in establishing itself as a leading nation in global AI convergence research and industry. To achieve this, we will spare no effort in providing the best research environment and active support.”
KAIST plans to actively pursue the InnoCORE project to secure global competitiveness in AI convergence research and contribute to the development of advanced industries. The eight selected research groups will finalize their detailed research plans by the end of June and commence full-scale research in July.
2025.06.19 View 2698
‘InnoCORE Research Group’ Launched to Lead AI Convergence Innovation
KAIST announced on the 16th of June that it has launched the ‘InnoCORE (Innovation-Core) Research Group,’ which will lead advanced strategic research in AI convergence (AI+S&T), in cooperation with the Ministry of Science and ICT (Minister Yoo Sang-im, hereinafter referred to as MSIT) and DGIST, GIST, and UNIST*. Through this, the group plans to actively recruit up to 200 world-class postdoctoral researchers.
DGIST (Daegu Gyeongbuk Institute of Science & Technology), GIST (Gwangju Institute of Science & Technology), UNIST (Ulsan National Institute of Science and Technology)
The ‘InnoCORE Research Group’ aims to foster core research personnel who will lead innovation in the field of AI convergence, focusing on nurturing and attracting high-level research talent in AI+Science & Technology. This is a strategic response to prevent brain drain of domestic talent and attract excellent overseas talent amidst the accelerating global competition for AI talent.
Through this initiative, our university plans to accelerate AI-based science and technology innovation and disseminate research achievements across industries and the economy by supporting top domestic and international postdoctoral researchers to dedicate themselves to developing AI convergence technologies in an advanced collaborative research environment.
The InnoCORE project for advanced AI+S&T convergence research and global talent attraction is jointly promoted by four science and technology institutes, including KAIST. It is structured around AI core technologies (such as hyper-scale language models, AI semiconductors) and AI convergence technologies (such as bio, manufacturing, energy, and aerospace).
As the leading institution, our university operates the following four research groups:
Hyper-scale Language Model Innovation Research Group: Advancement of LLM technology and research on generative AI, multimodal AI, and ensuring reliability.
AI-based Intelligent Design-Manufacturing Integration Research Group: Establishment of an AI platform for the entire lifecycle of the manufacturing industry and innovation in design and processes.
AI-Innovation Drug Research Group: Securing AI-based drug development technologies across the entire lifecycle and overcoming intractable diseases.
AI-Transformed Aerospace Research Group: AI transformation of aerospace systems throughout their lifecycle and development of new technologies such as autonomous flight and space communication.
< Poster on the InnoCORE Global Jobfair for Recruitment of Postdoctoral Researchers >
In addition, a total of eight research groups are formed to promote global collaborative convergence research, including those led by DGIST, GIST, and UNIST: ▲Bio-Integrated Physical AI, ▲Early Diagnosis of Brain Diseases AI+Nano Convergence, ▲Intelligent Hydrogen Technology Innovation, and ▲AI-Space Solar Power Research Group.
Starting in 2025, the four science and technology institutes, including KAIST, will officially begin recruiting 400 postdoctoral researchers in the AI+S&T fields. Selected postdoctoral researchers will be guaranteed high-level treatment with an annual salary of over 90 million KRW, and additional support through matching with companies and research projects is also planned.
In particular, global recruitment fairs will be held in major US regions to expand the attraction of excellent overseas talent. Local recruitment fairs will be held in Boston (Harvard, MIT), New York (NYU), and Silicon Valley (Stanford) in June, along with promotions through global academic journals such as Nature and Science, and LinkedIn.
KAIST plans to provide multiple mentor programs, global joint research opportunities, and excellent infrastructure (such as supercomputers, semiconductor fabs, and AI research platforms) within the research groups to enable postdoctoral researchers to collaborate with experts from various academic and industrial fields.
President Kwang Hyung Lee emphasized, “Through this InnoCORE project, KAIST will leap forward as a Global Hub for AI+S&T convergence research. Young researchers from around the world will challenge themselves and grow at KAIST, and our country will play a pivotal role in establishing itself as a leading nation in global AI convergence research and industry. To achieve this, we will spare no effort in providing the best research environment and active support.”
KAIST plans to actively pursue the InnoCORE project to secure global competitiveness in AI convergence research and contribute to the development of advanced industries. The eight selected research groups will finalize their detailed research plans by the end of June and commence full-scale research in July.
2025.06.19 View 2698 -
 KAIST Successfully Develops High-Performance Water Electrolysis Without Platinum, Bringing Hydrogen Economy Closer
< Photo 1. (Front row, from left) Jeesoo Park (Ph.D. Candidate), Professor Hee-Tak Kim (Back row, from left) Kyunghwa Seok (Ph.D. Candidate), Dr. Gisu Doo, Euntaek Oh (Ph.D. Candidate) >
Hydrogen is gaining attention as a clean energy source that emits no carbon. Among various methods, water electrolysis, which splits water into hydrogen and oxygen using electricity, is recognized as an eco-friendly hydrogen production method. Specifically, proton exchange membrane water electrolysis (PEMWE) is considered a next-generation hydrogen production technology due to its ability to produce high-purity hydrogen at high pressure. However, existing PEMWE technology has faced limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coating materials. Korean researchers have now proposed a new solution to address these technical and economic bottlenecks.
KAIST (President Kwang Hyung Lee) announced on June 11th that a research team led by Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering, in a joint study with Dr. Gisu Doo of the Korea Institute of Energy Research (KIER, President Chang-keun Lee), has developed a next-generation water electrolysis technology that achieves high performance without the need for expensive platinum (Pt) coating.
The research team focused on the primary reason why 'iridium oxide (IrOx),' a highly active catalyst for water electrolysis electrodes, fails to perform optimally. They found that this is due to inefficient electron transfer and, for the first time in the world, demonstrated that performance can be maximized simply by controlling the catalyst particle size.
In this study, it was revealed that the reason iridium oxide catalysts do not exhibit excellent performance without platinum coating is due to 'electron transport resistance' that occurs at the interface between the catalyst, the ion conductor (hereinafter referred to as ionomer), and the Ti (titanium) substrate—core components inherently used together in water electrolysis electrodes.
Specifically, they identified that the 'pinch-off' phenomenon, where the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the critical cause of reduced conductivity. The ionomer has properties close to an electron insulator, thereby hindering electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium substrate, significantly increasing resistance.
< Figure 1. Infographic related to electron transport resistance at the catalyst layer/diffusion layer interface >
To address this, the research team fabricated and compared catalysts of various particle sizes. Through single-cell evaluation and multiphysics simulations, they demonstrated, for the first time globally, that when iridium oxide catalyst particles with a size of 20 nanometers (nm) or larger are used, the ionomer mixed region decreases, ensuring an electron pathway and restoring conductivity.
Moreover, they successfully optimized the interfacial structure through precise design, simultaneously ensuring both reactivity and electron transport. This achievement demonstrated that the previously unavoidable trade-off between catalyst activity and conductivity can be overcome through meticulous interfacial design.
This breakthrough is expected to be a significant milestone not only for the development of high-performance catalyst materials but also for the future commercialization of proton exchange membrane water electrolysis systems that can achieve high efficiency while drastically reducing the amount of precious metals used.
Professor Hee-Tak Kim stated, "This research presents a new interface design strategy that can resolve the interfacial conductivity problem, which was a bottleneck in high-performance water electrolysis technology." He added, "By securing high performance even without expensive materials like platinum, it will be a stepping stone closer to realizing a hydrogen economy."
This research, with Jeesoo Park, a Ph.D. student from the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7th in 'Energy & Environmental Science' (IF: 32.4, 2025), a leading international journal in the energy and environmental fields, and was recognized for its innovativeness and impact. (Paper title: On the interface electron transport problem of highly active IrOx catalysts, DOI: 10.1039/D4EE05816J).
This research was supported by the New and Renewable Energy Core Technology Development Project of the Ministry of Trade, Industry and Energy.
2025.06.11 View 3313
KAIST Successfully Develops High-Performance Water Electrolysis Without Platinum, Bringing Hydrogen Economy Closer
< Photo 1. (Front row, from left) Jeesoo Park (Ph.D. Candidate), Professor Hee-Tak Kim (Back row, from left) Kyunghwa Seok (Ph.D. Candidate), Dr. Gisu Doo, Euntaek Oh (Ph.D. Candidate) >
Hydrogen is gaining attention as a clean energy source that emits no carbon. Among various methods, water electrolysis, which splits water into hydrogen and oxygen using electricity, is recognized as an eco-friendly hydrogen production method. Specifically, proton exchange membrane water electrolysis (PEMWE) is considered a next-generation hydrogen production technology due to its ability to produce high-purity hydrogen at high pressure. However, existing PEMWE technology has faced limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coating materials. Korean researchers have now proposed a new solution to address these technical and economic bottlenecks.
KAIST (President Kwang Hyung Lee) announced on June 11th that a research team led by Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering, in a joint study with Dr. Gisu Doo of the Korea Institute of Energy Research (KIER, President Chang-keun Lee), has developed a next-generation water electrolysis technology that achieves high performance without the need for expensive platinum (Pt) coating.
The research team focused on the primary reason why 'iridium oxide (IrOx),' a highly active catalyst for water electrolysis electrodes, fails to perform optimally. They found that this is due to inefficient electron transfer and, for the first time in the world, demonstrated that performance can be maximized simply by controlling the catalyst particle size.
In this study, it was revealed that the reason iridium oxide catalysts do not exhibit excellent performance without platinum coating is due to 'electron transport resistance' that occurs at the interface between the catalyst, the ion conductor (hereinafter referred to as ionomer), and the Ti (titanium) substrate—core components inherently used together in water electrolysis electrodes.
Specifically, they identified that the 'pinch-off' phenomenon, where the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the critical cause of reduced conductivity. The ionomer has properties close to an electron insulator, thereby hindering electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium substrate, significantly increasing resistance.
< Figure 1. Infographic related to electron transport resistance at the catalyst layer/diffusion layer interface >
To address this, the research team fabricated and compared catalysts of various particle sizes. Through single-cell evaluation and multiphysics simulations, they demonstrated, for the first time globally, that when iridium oxide catalyst particles with a size of 20 nanometers (nm) or larger are used, the ionomer mixed region decreases, ensuring an electron pathway and restoring conductivity.
Moreover, they successfully optimized the interfacial structure through precise design, simultaneously ensuring both reactivity and electron transport. This achievement demonstrated that the previously unavoidable trade-off between catalyst activity and conductivity can be overcome through meticulous interfacial design.
This breakthrough is expected to be a significant milestone not only for the development of high-performance catalyst materials but also for the future commercialization of proton exchange membrane water electrolysis systems that can achieve high efficiency while drastically reducing the amount of precious metals used.
Professor Hee-Tak Kim stated, "This research presents a new interface design strategy that can resolve the interfacial conductivity problem, which was a bottleneck in high-performance water electrolysis technology." He added, "By securing high performance even without expensive materials like platinum, it will be a stepping stone closer to realizing a hydrogen economy."
This research, with Jeesoo Park, a Ph.D. student from the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7th in 'Energy & Environmental Science' (IF: 32.4, 2025), a leading international journal in the energy and environmental fields, and was recognized for its innovativeness and impact. (Paper title: On the interface electron transport problem of highly active IrOx catalysts, DOI: 10.1039/D4EE05816J).
This research was supported by the New and Renewable Energy Core Technology Development Project of the Ministry of Trade, Industry and Energy.
2025.06.11 View 3313 -
 KAIST Professor Jee-Hwan Ryu Receives Global IEEE Robotics Journal Best Paper Award
- Professor Jee-Hwan Ryu of Civil and Environmental Engineering receives the Best Paper Award from the Institute of Electrical and Electronics Engineers (IEEE) Robotics Journal, officially presented at ICRA, a world-renowned robotics conference.
- This is the highest level of international recognition, awarded to only the top 5 papers out of approximately 1,500 published in 2024.
- Securing a new working channel technology for soft growing robots expands the practicality and application possibilities in the field of soft robotics.
< Professor Jee-Hwan Ryu (left), Nam Gyun Kim, Ph.D. Candidate (right) from the KAIST Department of Civil and Environmental Engineering and KAIST Robotics Program >
KAIST (President Kwang-Hyung Lee) announced on the 6th that Professor Jee-Hwan Ryu from the Department of Civil and Environmental Engineering received the 2024 Best Paper Award from the Robotics and Automation Letters (RA-L), a premier journal under the IEEE, at the '2025 IEEE International Conference on Robotics and Automation (ICRA)' held in Atlanta, USA, on May 22nd.
This Best Paper Award is a prestigious honor presented to only the top 5 papers out of approximately 1,500 published in 2024, boasting high international competition and authority.
The award-winning paper by Professor Ryu proposes a novel working channel securing mechanism that significantly expands the practicality and application possibilities of 'Soft Growing Robots,' which are based on soft materials that move or perform tasks through a growing motion similar to plant roots.
< IEEE Robotics Journal Award Ceremony >
Existing soft growing robots move by inflating or contracting their bodies through increasing or decreasing internal pressure, which can lead to blockages in their internal passages. In contrast, the newly developed soft growing robot achieves a growing function while maintaining the internal passage pressure equal to the external atmospheric pressure, thereby successfully securing an internal passage while retaining the robot's flexible and soft characteristics.
This structure allows various materials or tools to be freely delivered through the internal passage (working channel) within the robot and offers the advantage of performing multi-purpose tasks by flexibly replacing equipment according to the working environment.
The research team fabricated a prototype to prove the effectiveness of this technology and verified its performance through various experiments. Specifically, in the slide plate experiment, they confirmed whether materials or equipment could pass through the robot's internal channel without obstruction, and in the pipe pulling experiment, they verified if a long pipe-shaped tool could be pulled through the internal channel.
< Figure 1. Overall hardware structure of the proposed soft growing robot (left) and a cross-sectional view composing the inflatable structure (right) >
Experimental results demonstrated that the internal channel remained stable even while the robot was growing, serving as a key basis for supporting the technology's practicality and scalability.
Professor Jee-Hwan Ryu stated, "This award is very meaningful as it signifies the global recognition of Korea's robotics technology and academic achievements. Especially, it holds great significance in achieving technical progress that can greatly expand the practicality and application fields of soft growing robots. This achievement was possible thanks to the dedication and collaboration of the research team, and I will continue to contribute to the development of robotics technology through innovative research."
< Figure 2. Material supplying mechanism of the Soft Growing Robot >
This research was co-authored by Dongoh Seo, Ph.D. Candidate in Civil and Environmental Engineering, and Nam Gyun Kim, Ph.D. Candidate in Robotics. It was published in IEEE Robotics and Automation Letters on September 1, 2024.
(Paper Title: Inflatable-Structure-Based Working-Channel Securing Mechanism for Soft Growing Robots, DOI: 10.1109/LRA.2024.3426322)
This project was supported simultaneously by the National Research Foundation of Korea's Future Promising Convergence Technology Pioneer Research Project and Mid-career Researcher Project.
2025.06.09 View 3435
KAIST Professor Jee-Hwan Ryu Receives Global IEEE Robotics Journal Best Paper Award
- Professor Jee-Hwan Ryu of Civil and Environmental Engineering receives the Best Paper Award from the Institute of Electrical and Electronics Engineers (IEEE) Robotics Journal, officially presented at ICRA, a world-renowned robotics conference.
- This is the highest level of international recognition, awarded to only the top 5 papers out of approximately 1,500 published in 2024.
- Securing a new working channel technology for soft growing robots expands the practicality and application possibilities in the field of soft robotics.
< Professor Jee-Hwan Ryu (left), Nam Gyun Kim, Ph.D. Candidate (right) from the KAIST Department of Civil and Environmental Engineering and KAIST Robotics Program >
KAIST (President Kwang-Hyung Lee) announced on the 6th that Professor Jee-Hwan Ryu from the Department of Civil and Environmental Engineering received the 2024 Best Paper Award from the Robotics and Automation Letters (RA-L), a premier journal under the IEEE, at the '2025 IEEE International Conference on Robotics and Automation (ICRA)' held in Atlanta, USA, on May 22nd.
This Best Paper Award is a prestigious honor presented to only the top 5 papers out of approximately 1,500 published in 2024, boasting high international competition and authority.
The award-winning paper by Professor Ryu proposes a novel working channel securing mechanism that significantly expands the practicality and application possibilities of 'Soft Growing Robots,' which are based on soft materials that move or perform tasks through a growing motion similar to plant roots.
< IEEE Robotics Journal Award Ceremony >
Existing soft growing robots move by inflating or contracting their bodies through increasing or decreasing internal pressure, which can lead to blockages in their internal passages. In contrast, the newly developed soft growing robot achieves a growing function while maintaining the internal passage pressure equal to the external atmospheric pressure, thereby successfully securing an internal passage while retaining the robot's flexible and soft characteristics.
This structure allows various materials or tools to be freely delivered through the internal passage (working channel) within the robot and offers the advantage of performing multi-purpose tasks by flexibly replacing equipment according to the working environment.
The research team fabricated a prototype to prove the effectiveness of this technology and verified its performance through various experiments. Specifically, in the slide plate experiment, they confirmed whether materials or equipment could pass through the robot's internal channel without obstruction, and in the pipe pulling experiment, they verified if a long pipe-shaped tool could be pulled through the internal channel.
< Figure 1. Overall hardware structure of the proposed soft growing robot (left) and a cross-sectional view composing the inflatable structure (right) >
Experimental results demonstrated that the internal channel remained stable even while the robot was growing, serving as a key basis for supporting the technology's practicality and scalability.
Professor Jee-Hwan Ryu stated, "This award is very meaningful as it signifies the global recognition of Korea's robotics technology and academic achievements. Especially, it holds great significance in achieving technical progress that can greatly expand the practicality and application fields of soft growing robots. This achievement was possible thanks to the dedication and collaboration of the research team, and I will continue to contribute to the development of robotics technology through innovative research."
< Figure 2. Material supplying mechanism of the Soft Growing Robot >
This research was co-authored by Dongoh Seo, Ph.D. Candidate in Civil and Environmental Engineering, and Nam Gyun Kim, Ph.D. Candidate in Robotics. It was published in IEEE Robotics and Automation Letters on September 1, 2024.
(Paper Title: Inflatable-Structure-Based Working-Channel Securing Mechanism for Soft Growing Robots, DOI: 10.1109/LRA.2024.3426322)
This project was supported simultaneously by the National Research Foundation of Korea's Future Promising Convergence Technology Pioneer Research Project and Mid-career Researcher Project.
2025.06.09 View 3435