Ro
-
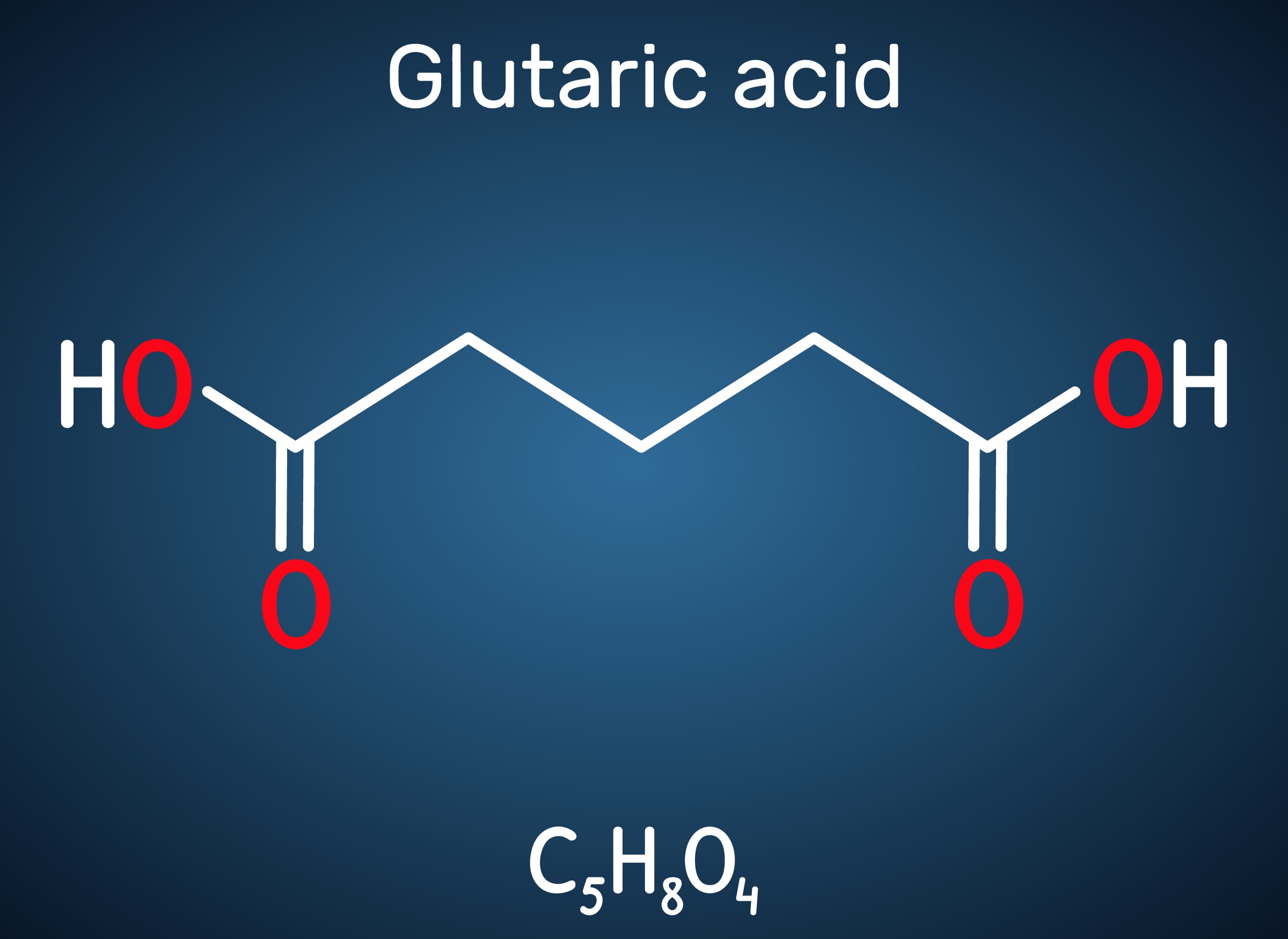 Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 8502
Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 8502 -
 Professor Kyu-Young Whang Donates Toward the 50th Anniversary Memorial Building
Distinguished Professor Kyu-Young Whang from the School of Computing made a gift of 100 million KRW toward the construction of the 50th Anniversary Memorial Building during a ceremony on November 3 at the Daejeon campus. "As a member of the first class of KAIST, I feel very delighted to play a part in the fundraising campaign for the 50th anniversary celebration. This is also a token of appreciation to my alma mater and I look forward to alumni and the KAIST community joining this campaign," said Professor Emeritus Whang. KAIST will name the Kyu-Young Whang and Jonghae Song Christian Seminar Room at the 50th Anniversary Memorial Building. The ground will be broken in 2022 for construction of the building.
2020.11.04 View 7819
Professor Kyu-Young Whang Donates Toward the 50th Anniversary Memorial Building
Distinguished Professor Kyu-Young Whang from the School of Computing made a gift of 100 million KRW toward the construction of the 50th Anniversary Memorial Building during a ceremony on November 3 at the Daejeon campus. "As a member of the first class of KAIST, I feel very delighted to play a part in the fundraising campaign for the 50th anniversary celebration. This is also a token of appreciation to my alma mater and I look forward to alumni and the KAIST community joining this campaign," said Professor Emeritus Whang. KAIST will name the Kyu-Young Whang and Jonghae Song Christian Seminar Room at the 50th Anniversary Memorial Building. The ground will be broken in 2022 for construction of the building.
2020.11.04 View 7819 -
 Chemical Scissors Snip 2D Transition Metal Dichalcogenides into Nanoribbon
New ‘nanoribbon’ catalyst should slash cost of hydrogen production for clean fuels
Researchers have identified a potential catalyst alternative – and an innovative way to produce them using chemical ‘scissors’ – that could make hydrogen production more economical.
The research team led by Professor Sang Ouk Kim at the Department of Materials Science and Engineering published their work in Nature Communications.
Hydrogen is likely to play a key role in the clean transition away from fossil fuels and other processes that produce greenhouse gas emissions. There is a raft of transportation sectors such as long-haul shipping and aviation that are difficult to electrify and so will require cleanly produced hydrogen as a fuel or as a feedstock for other carbon-neutral synthetic fuels. Likewise, fertilizer production and the steel sector are unlikely to be “de-carbonized” without cheap and clean hydrogen.
The problem is that the cheapest methods by far of producing hydrogen gas is currently from natural gas, a process that itself produces the greenhouse gas carbon dioxide–which defeats the purpose.
Alternative techniques of hydrogen production, such as electrolysis using an electric current between two electrodes plunged into water to overcome the chemical bonds holding water together, thereby splitting it into its constituent elements, oxygen and hydrogen are very well established. But one of the factors contributing to the high cost, beyond being extremely energy-intensive, is the need for the very expensive precious and relatively rare metal platinum. The platinum is used as a catalyst–a substance that kicks off or speeds up a chemical reaction–in the hydrogen production process.
As a result, researchers have long been on the hunt for a substitution for platinum -- another catalyst that is abundant in the earth and thus much cheaper.
Transition metal dichalcogenides, or TMDs, in a nanomaterial form, have for some time been considered a good candidate as a catalyst replacement for platinum. These are substances composed of one atom of a transition metal (the elements in the middle part of the periodic table) and two atoms of a chalcogen element (the elements in the third-to-last column in the periodic table, specifically sulfur, selenium and tellurium).
What makes TMDs a good bet as a platinum replacement is not just that they are much more abundant, but also their electrons are structured in a way that gives the electrodes a boost.
In addition, a TMD that is a nanomaterial is essentially a two-dimensional super-thin sheet only a few atoms thick, just like graphene. The ultrathin nature of a 2-D TMD nanosheet allows for a great many more TMD molecules to be exposed during the catalysis process than would be the case in a block of the stuff, thus kicking off and speeding up the hydrogen-making chemical reaction that much more.
However, even here the TMD molecules are only reactive at the four edges of a nanosheet. In the flat interior, not much is going on. In order to increase the chemical reaction rate in the production of hydrogen, the nanosheet would need to be cut into very thin – almost one-dimensional strips, thereby creating many edges.
In response, the research team developed what are in essence a pair of chemical scissors that can snip TMD into tiny strips.
“Up to now, the only substances that anyone has been able to turn into these ‘nano-ribbons’ are graphene and phosphorene,” said Sang Professor Kim, one of the researchers involved in devising the process.
“But they’re both made up of just one element, so it’s pretty straightforward. Figuring out how to do it for TMD, which is made of two elements was going to be much harder.”
The ‘scissors’ involve a two-step process involving first inserting lithium ions into the layered structure of the TMD sheets, and then using ultrasound to cause a spontaneous ‘unzipping’ in straight lines.
“It works sort of like how when you split a plank of plywood: it breaks easily in one direction along the grain,” Professor Kim continued. “It’s actually really simple.”
The researchers then tried it with various types of TMDs, including those made of molybdenum, selenium, sulfur, tellurium and tungsten. All worked just as well, with a catalytic efficiency as effective as platinum’s.
Because of the simplicity of the procedure, this method should be able to be used not just in the large-scale production of TMD nanoribbons, but also to make similar nanoribbons from other multi-elemental 2D materials for purposes beyond just hydrogen production.
-ProfileProfessor Sang Ouk KimSoft Nanomaterials Laboratory (http://snml.kaist.ac.kr)Department of Materials Science and EngineeringKAIST
2020.10.29 View 9880
Chemical Scissors Snip 2D Transition Metal Dichalcogenides into Nanoribbon
New ‘nanoribbon’ catalyst should slash cost of hydrogen production for clean fuels
Researchers have identified a potential catalyst alternative – and an innovative way to produce them using chemical ‘scissors’ – that could make hydrogen production more economical.
The research team led by Professor Sang Ouk Kim at the Department of Materials Science and Engineering published their work in Nature Communications.
Hydrogen is likely to play a key role in the clean transition away from fossil fuels and other processes that produce greenhouse gas emissions. There is a raft of transportation sectors such as long-haul shipping and aviation that are difficult to electrify and so will require cleanly produced hydrogen as a fuel or as a feedstock for other carbon-neutral synthetic fuels. Likewise, fertilizer production and the steel sector are unlikely to be “de-carbonized” without cheap and clean hydrogen.
The problem is that the cheapest methods by far of producing hydrogen gas is currently from natural gas, a process that itself produces the greenhouse gas carbon dioxide–which defeats the purpose.
Alternative techniques of hydrogen production, such as electrolysis using an electric current between two electrodes plunged into water to overcome the chemical bonds holding water together, thereby splitting it into its constituent elements, oxygen and hydrogen are very well established. But one of the factors contributing to the high cost, beyond being extremely energy-intensive, is the need for the very expensive precious and relatively rare metal platinum. The platinum is used as a catalyst–a substance that kicks off or speeds up a chemical reaction–in the hydrogen production process.
As a result, researchers have long been on the hunt for a substitution for platinum -- another catalyst that is abundant in the earth and thus much cheaper.
Transition metal dichalcogenides, or TMDs, in a nanomaterial form, have for some time been considered a good candidate as a catalyst replacement for platinum. These are substances composed of one atom of a transition metal (the elements in the middle part of the periodic table) and two atoms of a chalcogen element (the elements in the third-to-last column in the periodic table, specifically sulfur, selenium and tellurium).
What makes TMDs a good bet as a platinum replacement is not just that they are much more abundant, but also their electrons are structured in a way that gives the electrodes a boost.
In addition, a TMD that is a nanomaterial is essentially a two-dimensional super-thin sheet only a few atoms thick, just like graphene. The ultrathin nature of a 2-D TMD nanosheet allows for a great many more TMD molecules to be exposed during the catalysis process than would be the case in a block of the stuff, thus kicking off and speeding up the hydrogen-making chemical reaction that much more.
However, even here the TMD molecules are only reactive at the four edges of a nanosheet. In the flat interior, not much is going on. In order to increase the chemical reaction rate in the production of hydrogen, the nanosheet would need to be cut into very thin – almost one-dimensional strips, thereby creating many edges.
In response, the research team developed what are in essence a pair of chemical scissors that can snip TMD into tiny strips.
“Up to now, the only substances that anyone has been able to turn into these ‘nano-ribbons’ are graphene and phosphorene,” said Sang Professor Kim, one of the researchers involved in devising the process.
“But they’re both made up of just one element, so it’s pretty straightforward. Figuring out how to do it for TMD, which is made of two elements was going to be much harder.”
The ‘scissors’ involve a two-step process involving first inserting lithium ions into the layered structure of the TMD sheets, and then using ultrasound to cause a spontaneous ‘unzipping’ in straight lines.
“It works sort of like how when you split a plank of plywood: it breaks easily in one direction along the grain,” Professor Kim continued. “It’s actually really simple.”
The researchers then tried it with various types of TMDs, including those made of molybdenum, selenium, sulfur, tellurium and tungsten. All worked just as well, with a catalytic efficiency as effective as platinum’s.
Because of the simplicity of the procedure, this method should be able to be used not just in the large-scale production of TMD nanoribbons, but also to make similar nanoribbons from other multi-elemental 2D materials for purposes beyond just hydrogen production.
-ProfileProfessor Sang Ouk KimSoft Nanomaterials Laboratory (http://snml.kaist.ac.kr)Department of Materials Science and EngineeringKAIST
2020.10.29 View 9880 -
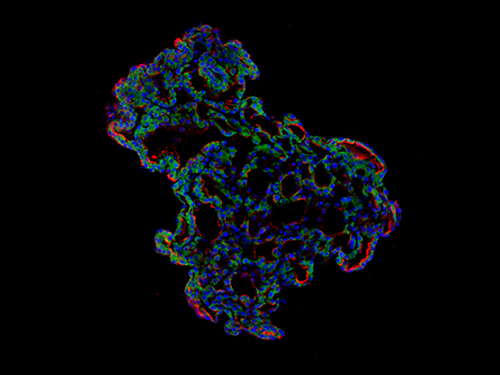 'Mini-Lungs' Reveal Early Stages of SARS-CoV-2 Infection
Researchers in Korea and the UK have successfully grown miniature models of critical lung structures called alveoli, and used them to study how the coronavirus that causes COVID-19 infects the lungs.
To date, there have been more than 40 million cases of COVID-19 and almost 1.13 million deaths worldwide. The main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be alveoli – tiny air sacs in the lungs that take up the oxygen we breathe and exchange it with carbon dioxide to exhale.
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST in collaboration with the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organizing alveolar-like 3D structures that mimic the behaviour of key lung tissue.
“The research community now has a powerful new platform to study precisely how the virus infects the lungs, as well as explore possible treatments,” said Professor Ju, co-senior author of the research.
Dr. Joo-Hyeon Lee, another co-senior author at the Wellcome-MRC Cambridge Stem Cell Institute, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in Korea who was diagnosed with COVID-19 on January 26 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Professor Ju. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr. Lee.
The research was a collaboration involving scientists from KAIST, the University of Cambridge, Korea National Institute of Health, Institute for Basic Science (IBS), Seoul National University Hospital and Genome Insight in Korea.
- ProfileProfessor Young Seok JuLaboratory of Cancer Genomics https://julab.kaist.ac.kr the Graduate School of Medical Science and EngineeringKAIST
2020.10.26 View 13427
'Mini-Lungs' Reveal Early Stages of SARS-CoV-2 Infection
Researchers in Korea and the UK have successfully grown miniature models of critical lung structures called alveoli, and used them to study how the coronavirus that causes COVID-19 infects the lungs.
To date, there have been more than 40 million cases of COVID-19 and almost 1.13 million deaths worldwide. The main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be alveoli – tiny air sacs in the lungs that take up the oxygen we breathe and exchange it with carbon dioxide to exhale.
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST in collaboration with the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organizing alveolar-like 3D structures that mimic the behaviour of key lung tissue.
“The research community now has a powerful new platform to study precisely how the virus infects the lungs, as well as explore possible treatments,” said Professor Ju, co-senior author of the research.
Dr. Joo-Hyeon Lee, another co-senior author at the Wellcome-MRC Cambridge Stem Cell Institute, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in Korea who was diagnosed with COVID-19 on January 26 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Professor Ju. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr. Lee.
The research was a collaboration involving scientists from KAIST, the University of Cambridge, Korea National Institute of Health, Institute for Basic Science (IBS), Seoul National University Hospital and Genome Insight in Korea.
- ProfileProfessor Young Seok JuLaboratory of Cancer Genomics https://julab.kaist.ac.kr the Graduate School of Medical Science and EngineeringKAIST
2020.10.26 View 13427 -
 E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 13046
E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 13046 -
 Deep Learning Helps Explore the Structural and Strategic Bases of Autism
Psychiatrists typically diagnose autism spectrum disorders (ASD) by observing a person’s behavior and by leaning on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), widely considered the “bible” of mental health diagnosis.
However, there are substantial differences amongst individuals on the spectrum and a great deal remains unknown by science about the causes of autism, or even what autism is. As a result, an accurate diagnosis of ASD and a prognosis prediction for patients can be extremely difficult.
But what if artificial intelligence (AI) could help? Deep learning, a type of AI, deploys artificial neural networks based on the human brain to recognize patterns in a way that is akin to, and in some cases can surpass, human ability. The technique, or rather suite of techniques, has enjoyed remarkable success in recent years in fields as diverse as voice recognition, translation, autonomous vehicles, and drug discovery.
A group of researchers from KAIST in collaboration with the Yonsei University College of Medicine has applied these deep learning techniques to autism diagnosis. Their findings were published on August 14 in the journal IEEE Access.
Magnetic resonance imaging (MRI) scans of brains of people known to have autism have been used by researchers and clinicians to try to identify structures of the brain they believed were associated with ASD. These researchers have achieved considerable success in identifying abnormal grey and white matter volume and irregularities in cerebral cortex activation and connections as being associated with the condition.
These findings have subsequently been deployed in studies attempting more consistent diagnoses of patients than has been achieved via psychiatrist observations during counseling sessions. While such studies have reported high levels of diagnostic accuracy, the number of participants in these studies has been small, often under 50, and diagnostic performance drops markedly when applied to large sample sizes or on datasets that include people from a wide variety of populations and locations.
“There was something as to what defines autism that human researchers and clinicians must have been overlooking,” said Keun-Ah Cheon, one of the two corresponding authors and a professor in Department of Child and Adolescent Psychiatry at Severance Hospital of the Yonsei University College of Medicine.
“And humans poring over thousands of MRI scans won’t be able to pick up on what we’ve been missing,” she continued. “But we thought AI might be able to.”
So the team applied five different categories of deep learning models to an open-source dataset of more than 1,000 MRI scans from the Autism Brain Imaging Data Exchange (ABIDE) initiative, which has collected brain imaging data from laboratories around the world, and to a smaller, but higher-resolution MRI image dataset (84 images) taken from the Child Psychiatric Clinic at Severance Hospital, Yonsei University College of Medicine. In both cases, the researchers used both structural MRIs (examining the anatomy of the brain) and functional MRIs (examining brain activity in different regions).
The models allowed the team to explore the structural bases of ASD brain region by brain region, focusing in particular on many structures below the cerebral cortex, including the basal ganglia, which are involved in motor function (movement) as well as learning and memory.
Crucially, these specific types of deep learning models also offered up possible explanations of how the AI had come up with its rationale for these findings.
“Understanding the way that the AI has classified these brain structures and dynamics is extremely important,” said Sang Wan Lee, the other corresponding author and an associate professor at KAIST. “It’s no good if a doctor can tell a patient that the computer says they have autism, but not be able to say why the computer knows that.”
The deep learning models were also able to describe how much a particular aspect contributed to ASD, an analysis tool that can assist psychiatric physicians during the diagnosis process to identify the severity of the autism.
“Doctors should be able to use this to offer a personalized diagnosis for patients, including a prognosis of how the condition could develop,” Lee said.
“Artificial intelligence is not going to put psychiatrists out of a job,” he explained. “But using AI as a tool should enable doctors to better understand and diagnose complex disorders than they could do on their own.”
-ProfileProfessor Sang Wan LeeDepartment of Bio and Brain EngineeringLaboratory for Brain and Machine Intelligence https://aibrain.kaist.ac.kr/
KAIST
2020.09.23 View 13010
Deep Learning Helps Explore the Structural and Strategic Bases of Autism
Psychiatrists typically diagnose autism spectrum disorders (ASD) by observing a person’s behavior and by leaning on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), widely considered the “bible” of mental health diagnosis.
However, there are substantial differences amongst individuals on the spectrum and a great deal remains unknown by science about the causes of autism, or even what autism is. As a result, an accurate diagnosis of ASD and a prognosis prediction for patients can be extremely difficult.
But what if artificial intelligence (AI) could help? Deep learning, a type of AI, deploys artificial neural networks based on the human brain to recognize patterns in a way that is akin to, and in some cases can surpass, human ability. The technique, or rather suite of techniques, has enjoyed remarkable success in recent years in fields as diverse as voice recognition, translation, autonomous vehicles, and drug discovery.
A group of researchers from KAIST in collaboration with the Yonsei University College of Medicine has applied these deep learning techniques to autism diagnosis. Their findings were published on August 14 in the journal IEEE Access.
Magnetic resonance imaging (MRI) scans of brains of people known to have autism have been used by researchers and clinicians to try to identify structures of the brain they believed were associated with ASD. These researchers have achieved considerable success in identifying abnormal grey and white matter volume and irregularities in cerebral cortex activation and connections as being associated with the condition.
These findings have subsequently been deployed in studies attempting more consistent diagnoses of patients than has been achieved via psychiatrist observations during counseling sessions. While such studies have reported high levels of diagnostic accuracy, the number of participants in these studies has been small, often under 50, and diagnostic performance drops markedly when applied to large sample sizes or on datasets that include people from a wide variety of populations and locations.
“There was something as to what defines autism that human researchers and clinicians must have been overlooking,” said Keun-Ah Cheon, one of the two corresponding authors and a professor in Department of Child and Adolescent Psychiatry at Severance Hospital of the Yonsei University College of Medicine.
“And humans poring over thousands of MRI scans won’t be able to pick up on what we’ve been missing,” she continued. “But we thought AI might be able to.”
So the team applied five different categories of deep learning models to an open-source dataset of more than 1,000 MRI scans from the Autism Brain Imaging Data Exchange (ABIDE) initiative, which has collected brain imaging data from laboratories around the world, and to a smaller, but higher-resolution MRI image dataset (84 images) taken from the Child Psychiatric Clinic at Severance Hospital, Yonsei University College of Medicine. In both cases, the researchers used both structural MRIs (examining the anatomy of the brain) and functional MRIs (examining brain activity in different regions).
The models allowed the team to explore the structural bases of ASD brain region by brain region, focusing in particular on many structures below the cerebral cortex, including the basal ganglia, which are involved in motor function (movement) as well as learning and memory.
Crucially, these specific types of deep learning models also offered up possible explanations of how the AI had come up with its rationale for these findings.
“Understanding the way that the AI has classified these brain structures and dynamics is extremely important,” said Sang Wan Lee, the other corresponding author and an associate professor at KAIST. “It’s no good if a doctor can tell a patient that the computer says they have autism, but not be able to say why the computer knows that.”
The deep learning models were also able to describe how much a particular aspect contributed to ASD, an analysis tool that can assist psychiatric physicians during the diagnosis process to identify the severity of the autism.
“Doctors should be able to use this to offer a personalized diagnosis for patients, including a prognosis of how the condition could develop,” Lee said.
“Artificial intelligence is not going to put psychiatrists out of a job,” he explained. “But using AI as a tool should enable doctors to better understand and diagnose complex disorders than they could do on their own.”
-ProfileProfessor Sang Wan LeeDepartment of Bio and Brain EngineeringLaboratory for Brain and Machine Intelligence https://aibrain.kaist.ac.kr/
KAIST
2020.09.23 View 13010 -
 Sturdy Fabric-Based Piezoelectric Energy Harvester Takes Us One Step Closer to Wearable Electronics
KAIST researchers presented a highly flexible but sturdy wearable piezoelectric harvester using the simple and easy fabrication process of hot pressing and tape casting. This energy harvester, which has record high interfacial adhesion strength, will take us one step closer to being able to manufacture embedded wearable electronics.
A research team led by Professor Seungbum Hong said that the novelty of this result lies in its simplicity, applicability, durability, and its new characterization of wearable electronic devices.
Wearable devices are increasingly being used in a wide array of applications from small electronics to embedded devices such as sensors, actuators, displays, and energy harvesters.
Despite their many advantages, high costs and complex fabrication processes remained challenges for reaching commercialization. In addition, their durability was frequently questioned. To address these issues, Professor Hong’s team developed a new fabrication process and analysis technology for testing the mechanical properties of affordable wearable devices.
For this process, the research team used a hot pressing and tape casting procedure to connect the fabric structures of polyester and a polymer film. Hot pressing has usually been used when making batteries and fuel cells due to its high adhesiveness. Above all, the process takes only two to three minutes.
The newly developed fabrication process will enable the direct application of a device into general garments using hot pressing just as graphic patches can be attached to garments using a heat press.
In particular, when the polymer film is hot pressed onto a fabric below its crystallization temperature, it transforms into an amorphous state. In this state, it compactly attaches to the concave surface of the fabric and infiltrates into the gaps between the transverse wefts and longitudinal warps. These features result in high interfacial adhesion strength. For this reason, hot pressing has the potential to reduce the cost of fabrication through the direct application of fabric-based wearable devices to common garments.
In addition to the conventional durability test of bending cycles, the newly introduced surface and interfacial cutting analysis system proved the high mechanical durability of the fabric-based wearable device by measuring the high interfacial adhesion strength between the fabric and the polymer film. Professor Hong said the study lays a new foundation for the manufacturing process and analysis of wearable devices using fabrics and polymers.
He added that his team first used the surface and interfacial cutting analysis system (SAICAS) in the field of wearable electronics to test the mechanical properties of polymer-based wearable devices. Their surface and interfacial cutting analysis system is more precise than conventional methods (peel test, tape test, and microstretch test) because it qualitatively and quantitatively measures the adhesion strength.
Professor Hong explained, “This study could enable the commercialization of highly durable wearable devices based on the analysis of their interfacial adhesion strength. Our study lays a new foundation for the manufacturing process and analysis of other devices using fabrics and polymers. We look forward to fabric-based wearable electronics hitting the market very soon.”
The results of this study were registered as a domestic patent in Korea last year, and published in Nano Energy this month. This study has been conducted through collaboration with Professor Yong Min Lee in the Department of Energy Science and Engineering at DGIST, Professor Kwangsoo No in the Department of Materials Science and Engineering at KAIST, and Professor Seunghwa Ryu in the Department of Mechanical Engineering at KAIST.
This study was supported by the High-Risk High-Return Project and the Global Singularity Research Project at KAIST, the National Research Foundation, and the Ministry of Science and ICT in Korea.
-Publication:
Jaegyu Kim, Seoungwoo Byun, Sangryun Lee, Jeongjae Ryu, Seongwoo Cho, Chungik Oh, Hongjun Kim, Kwangsoo No, Seunghwa Ryu, Yong Min Lee, Seungbum Hong*, Nano Energy 75 (2020), 104992.
https://doi.org/10.1016/j.nanoen.2020.104992
-Profile: Professor Seungbum Hong
seungbum@kaist.ac.kr
http://mii.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
2020.09.17 View 15665
Sturdy Fabric-Based Piezoelectric Energy Harvester Takes Us One Step Closer to Wearable Electronics
KAIST researchers presented a highly flexible but sturdy wearable piezoelectric harvester using the simple and easy fabrication process of hot pressing and tape casting. This energy harvester, which has record high interfacial adhesion strength, will take us one step closer to being able to manufacture embedded wearable electronics.
A research team led by Professor Seungbum Hong said that the novelty of this result lies in its simplicity, applicability, durability, and its new characterization of wearable electronic devices.
Wearable devices are increasingly being used in a wide array of applications from small electronics to embedded devices such as sensors, actuators, displays, and energy harvesters.
Despite their many advantages, high costs and complex fabrication processes remained challenges for reaching commercialization. In addition, their durability was frequently questioned. To address these issues, Professor Hong’s team developed a new fabrication process and analysis technology for testing the mechanical properties of affordable wearable devices.
For this process, the research team used a hot pressing and tape casting procedure to connect the fabric structures of polyester and a polymer film. Hot pressing has usually been used when making batteries and fuel cells due to its high adhesiveness. Above all, the process takes only two to three minutes.
The newly developed fabrication process will enable the direct application of a device into general garments using hot pressing just as graphic patches can be attached to garments using a heat press.
In particular, when the polymer film is hot pressed onto a fabric below its crystallization temperature, it transforms into an amorphous state. In this state, it compactly attaches to the concave surface of the fabric and infiltrates into the gaps between the transverse wefts and longitudinal warps. These features result in high interfacial adhesion strength. For this reason, hot pressing has the potential to reduce the cost of fabrication through the direct application of fabric-based wearable devices to common garments.
In addition to the conventional durability test of bending cycles, the newly introduced surface and interfacial cutting analysis system proved the high mechanical durability of the fabric-based wearable device by measuring the high interfacial adhesion strength between the fabric and the polymer film. Professor Hong said the study lays a new foundation for the manufacturing process and analysis of wearable devices using fabrics and polymers.
He added that his team first used the surface and interfacial cutting analysis system (SAICAS) in the field of wearable electronics to test the mechanical properties of polymer-based wearable devices. Their surface and interfacial cutting analysis system is more precise than conventional methods (peel test, tape test, and microstretch test) because it qualitatively and quantitatively measures the adhesion strength.
Professor Hong explained, “This study could enable the commercialization of highly durable wearable devices based on the analysis of their interfacial adhesion strength. Our study lays a new foundation for the manufacturing process and analysis of other devices using fabrics and polymers. We look forward to fabric-based wearable electronics hitting the market very soon.”
The results of this study were registered as a domestic patent in Korea last year, and published in Nano Energy this month. This study has been conducted through collaboration with Professor Yong Min Lee in the Department of Energy Science and Engineering at DGIST, Professor Kwangsoo No in the Department of Materials Science and Engineering at KAIST, and Professor Seunghwa Ryu in the Department of Mechanical Engineering at KAIST.
This study was supported by the High-Risk High-Return Project and the Global Singularity Research Project at KAIST, the National Research Foundation, and the Ministry of Science and ICT in Korea.
-Publication:
Jaegyu Kim, Seoungwoo Byun, Sangryun Lee, Jeongjae Ryu, Seongwoo Cho, Chungik Oh, Hongjun Kim, Kwangsoo No, Seunghwa Ryu, Yong Min Lee, Seungbum Hong*, Nano Energy 75 (2020), 104992.
https://doi.org/10.1016/j.nanoen.2020.104992
-Profile: Professor Seungbum Hong
seungbum@kaist.ac.kr
http://mii.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
2020.09.17 View 15665 -
 Life After COVID-19: Big Questions on Medical and Bio-Engineering
KAIST GSI forum explores big questions in the medical and bio-engineering revolution caused by the COVID-19 in fight against infectious diseases and life quality
On September 9, the Global Strategy Institute at KAIST will delve into innovative future strategies for the medical and bio-engineering sectors that have been disrupted by COVID-19. The forum will live stream via YouTube, KTV, and Naver TV from 9:00 am Korean time.
The online forum features a speaker lineup of world-renowned scholars who will discuss an array of bio-engineering technologies that will improve our quality of life and even extend our life span. This is the GSI’s third online forum since the first one in April that covered the socio-economic implications of the global pandemic and the second one in June focusing on the education sector.
In hosting the third round of the GSI Forum series, KAIST President Sung-Chul Shin stressed the power of science and technology saying, “In this world full of uncertainties, one thing for sure is that only the advancement of science and technology will deliver us from this crisis.” Korean Prime Minister Sye-Kyun Chung will also deliver a speech explaining the government’s response to COVID-19 and vaccine development strategies.
The President of the National Academy of Medicine in the US will share ideal policies to back up the bio-engineering and medical sectors and Futurist Thomas Frey from the Davinci Institute will present his distinct perspectives on our future lives after COVID-19. His thought-provoking insights on advancements in the bioengineering sector will examine whether humanity can put an end to infectious diseases and find new ways to lengthen our lives.
Two distinguished professors in the field of genetic engineering technology will share their latest breakthroughs. Professor George McDonald Church from Harvard Medical School who developed genome sequencing will deliver a keynote speech on how the advancement of gene editing and genome technology will overcome diseases and contribute to extending human life spans.
Professor Kwang-Soo Kim, a KAIST alumnus from Harvard Medical School who recently reported new discoveries for Parkinson’s disease treatment by reprogramming a patient’s own skin cells to replace cells in the brain, will introduce the latest clinical cell treatment technologies based on personalized therapeutics.
Senior Vice President and Chief Product Officer of Illumina Susan Tousi, a leading genome sequencing solution provider, will describe genome analysis technology and explore the potential for disease prevention.
KAIST medical scientist Jeong Ho Lee, who was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders. Professor Jin-Hyung Lee from Stanford University and Dr. David B. Resnik from the National Institute of Environmental Health Science will also join the speaker lineup to discuss genetics-based personalized solutions to extend human life spans.
The forum will also invite about 50 young scientists and medical researchers from around the world to participate in an online panel session. They will engage in a Q&A session and a discussion with the speakers.
(END)
2020.09.04 View 12302
Life After COVID-19: Big Questions on Medical and Bio-Engineering
KAIST GSI forum explores big questions in the medical and bio-engineering revolution caused by the COVID-19 in fight against infectious diseases and life quality
On September 9, the Global Strategy Institute at KAIST will delve into innovative future strategies for the medical and bio-engineering sectors that have been disrupted by COVID-19. The forum will live stream via YouTube, KTV, and Naver TV from 9:00 am Korean time.
The online forum features a speaker lineup of world-renowned scholars who will discuss an array of bio-engineering technologies that will improve our quality of life and even extend our life span. This is the GSI’s third online forum since the first one in April that covered the socio-economic implications of the global pandemic and the second one in June focusing on the education sector.
In hosting the third round of the GSI Forum series, KAIST President Sung-Chul Shin stressed the power of science and technology saying, “In this world full of uncertainties, one thing for sure is that only the advancement of science and technology will deliver us from this crisis.” Korean Prime Minister Sye-Kyun Chung will also deliver a speech explaining the government’s response to COVID-19 and vaccine development strategies.
The President of the National Academy of Medicine in the US will share ideal policies to back up the bio-engineering and medical sectors and Futurist Thomas Frey from the Davinci Institute will present his distinct perspectives on our future lives after COVID-19. His thought-provoking insights on advancements in the bioengineering sector will examine whether humanity can put an end to infectious diseases and find new ways to lengthen our lives.
Two distinguished professors in the field of genetic engineering technology will share their latest breakthroughs. Professor George McDonald Church from Harvard Medical School who developed genome sequencing will deliver a keynote speech on how the advancement of gene editing and genome technology will overcome diseases and contribute to extending human life spans.
Professor Kwang-Soo Kim, a KAIST alumnus from Harvard Medical School who recently reported new discoveries for Parkinson’s disease treatment by reprogramming a patient’s own skin cells to replace cells in the brain, will introduce the latest clinical cell treatment technologies based on personalized therapeutics.
Senior Vice President and Chief Product Officer of Illumina Susan Tousi, a leading genome sequencing solution provider, will describe genome analysis technology and explore the potential for disease prevention.
KAIST medical scientist Jeong Ho Lee, who was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders. Professor Jin-Hyung Lee from Stanford University and Dr. David B. Resnik from the National Institute of Environmental Health Science will also join the speaker lineup to discuss genetics-based personalized solutions to extend human life spans.
The forum will also invite about 50 young scientists and medical researchers from around the world to participate in an online panel session. They will engage in a Q&A session and a discussion with the speakers.
(END)
2020.09.04 View 12302 -
 Microscopy Approach Poised to Offer New Insights into Liver Diseases
Researchers have developed a new way to visualize the progression of nonalcoholic fatty liver disease (NAFLD) in mouse models of the disease. The new microscopy method provides a high-resolution 3D view that could lead to important new insights into NAFLD, a condition in which too much fat is stored in the liver.
“It is estimated that a quarter of the adult global population has NAFLD, yet an effective treatment strategy has not been found,” said professor Pilhan Kim from the Graduate School of Medical Science and Engineering at KAIST. “NAFLD is associated with obesity and type 2 diabetes and can sometimes progress to liver failure in serious case.”
In the Optical Society (OSA) journal Biomedical Optics Express, Professor Kim and colleagues reported their new imaging technique and showed that it can be used to observe how tiny droplets of fat, or lipids, accumulate in the liver cells of living mice over time.
“It has been challenging to find a treatment strategy for NAFLD because most studies examine excised liver tissue that represents just one timepoint in disease progression,” said Professor Kim. “Our technique can capture details of lipid accumulation over time, providing a highly useful research tool for identifying the multiple parameters that likely contribute to the disease and could be targeted with treatment.”
Capturing the dynamics of NAFLD in living mouse models of the disease requires the ability to observe quickly changing interactions of biological components in intact tissue in real-time. To accomplish this, the researchers developed a custom intravital confocal and two-photon microscopy system that acquires images of multiple fluorescent labels at video-rate with cellular resolution.
“With video-rate imaging capability, the continuous movement of liver tissue in live mice due to breathing and heart beating could be tracked in real time and precisely compensated,” said Professor Kim. “This provided motion-artifact free high-resolution images of cellular and sub-cellular sized individual lipid droplets.”
The key to fast imaging was a polygonal mirror that rotated at more than 240 miles per hour to provide extremely fast laser scanning. The researchers also incorporated four different lasers and four high-sensitivity optical detectors into the setup so that they could acquire multi-color images to capture different color fluorescent probes used to label the lipid droplets and microvasculature in the livers of live mice.
“Our approach can capture real-time changes in cell behavior and morphology, vascular structure and function, and the spatiotemporal localization of biological components while directly visualizing of lipid droplet development in NAFLD progression,” said Professor Kim. “It also allows the analysis of the highly complex behaviors of various immune cells as NAFLD progresses.”
The researchers demonstrated their approach by using it to observe the development and spatial distribution of lipid droplets in individual mice with NAFLD induced by a methionine and choline-deficient diet. Next, they plan to use it to study how the liver microenvironment changes during NAFLD progression by imaging the same mouse over time. They also want to use their microscope technique to visualize various immune cells and lipid droplets to better understand the complex liver microenvironment in NAFLD progression.
2020.08.21 View 11157
Microscopy Approach Poised to Offer New Insights into Liver Diseases
Researchers have developed a new way to visualize the progression of nonalcoholic fatty liver disease (NAFLD) in mouse models of the disease. The new microscopy method provides a high-resolution 3D view that could lead to important new insights into NAFLD, a condition in which too much fat is stored in the liver.
“It is estimated that a quarter of the adult global population has NAFLD, yet an effective treatment strategy has not been found,” said professor Pilhan Kim from the Graduate School of Medical Science and Engineering at KAIST. “NAFLD is associated with obesity and type 2 diabetes and can sometimes progress to liver failure in serious case.”
In the Optical Society (OSA) journal Biomedical Optics Express, Professor Kim and colleagues reported their new imaging technique and showed that it can be used to observe how tiny droplets of fat, or lipids, accumulate in the liver cells of living mice over time.
“It has been challenging to find a treatment strategy for NAFLD because most studies examine excised liver tissue that represents just one timepoint in disease progression,” said Professor Kim. “Our technique can capture details of lipid accumulation over time, providing a highly useful research tool for identifying the multiple parameters that likely contribute to the disease and could be targeted with treatment.”
Capturing the dynamics of NAFLD in living mouse models of the disease requires the ability to observe quickly changing interactions of biological components in intact tissue in real-time. To accomplish this, the researchers developed a custom intravital confocal and two-photon microscopy system that acquires images of multiple fluorescent labels at video-rate with cellular resolution.
“With video-rate imaging capability, the continuous movement of liver tissue in live mice due to breathing and heart beating could be tracked in real time and precisely compensated,” said Professor Kim. “This provided motion-artifact free high-resolution images of cellular and sub-cellular sized individual lipid droplets.”
The key to fast imaging was a polygonal mirror that rotated at more than 240 miles per hour to provide extremely fast laser scanning. The researchers also incorporated four different lasers and four high-sensitivity optical detectors into the setup so that they could acquire multi-color images to capture different color fluorescent probes used to label the lipid droplets and microvasculature in the livers of live mice.
“Our approach can capture real-time changes in cell behavior and morphology, vascular structure and function, and the spatiotemporal localization of biological components while directly visualizing of lipid droplet development in NAFLD progression,” said Professor Kim. “It also allows the analysis of the highly complex behaviors of various immune cells as NAFLD progresses.”
The researchers demonstrated their approach by using it to observe the development and spatial distribution of lipid droplets in individual mice with NAFLD induced by a methionine and choline-deficient diet. Next, they plan to use it to study how the liver microenvironment changes during NAFLD progression by imaging the same mouse over time. They also want to use their microscope technique to visualize various immune cells and lipid droplets to better understand the complex liver microenvironment in NAFLD progression.
2020.08.21 View 11157 -
 Deep Learning-Based Cough Recognition Model Helps Detect the Location of Coughing Sounds in Real Time
The Center for Noise and Vibration Control at KAIST announced that their coughing detection camera recognizes where coughing happens, visualizing the locations. The resulting cough recognition camera can track and record information about the person who coughed, their location, and the number of coughs on a real-time basis.
Professor Yong-Hwa Park from the Department of Mechanical Engineering developed a deep learning-based cough recognition model to classify a coughing sound in real time. The coughing event classification model is combined with a sound camera that visualizes their locations in public places. The research team said they achieved a best test accuracy of 87.4 %.
Professor Park said that it will be useful medical equipment during epidemics in public places such as schools, offices, and restaurants, and to constantly monitor patients’ conditions in a hospital room.
Fever and coughing are the most relevant respiratory disease symptoms, among which fever can be recognized remotely using thermal cameras. This new technology is expected to be very helpful for detecting epidemic transmissions in a non-contact way. The cough event classification model is combined with a sound camera that visualizes the cough event and indicates the location in the video image.
To develop a cough recognition model, a supervised learning was conducted with a convolutional neural network (CNN). The model performs binary classification with an input of a one-second sound profile feature, generating output to be either a cough event or something else.
In the training and evaluation, various datasets were collected from Audioset, DEMAND, ETSI, and TIMIT. Coughing and others sounds were extracted from Audioset, and the rest of the datasets were used as background noises for data augmentation so that this model could be generalized for various background noises in public places.
The dataset was augmented by mixing coughing sounds and other sounds from Audioset and background noises with the ratio of 0.15 to 0.75, then the overall volume was adjusted to 0.25 to 1.0 times to generalize the model for various distances.
The training and evaluation datasets were constructed by dividing the augmented dataset by 9:1, and the test dataset was recorded separately in a real office environment.
In the optimization procedure of the network model, training was conducted with various combinations of five acoustic features including spectrogram, Mel-scaled spectrogram and Mel-frequency cepstrum coefficients with seven optimizers. The performance of each combination was compared with the test dataset. The best test accuracy of 87.4% was achieved with Mel-scaled Spectrogram as the acoustic feature and ASGD as the optimizer.
The trained cough recognition model was combined with a sound camera. The sound camera is composed of a microphone array and a camera module. A beamforming process is applied to a collected set of acoustic data to find out the direction of incoming sound source. The integrated cough recognition model determines whether the sound is cough or not. If it is, the location of cough is visualized as a contour image with a ‘cough’ label at the location of the coughing sound source in a video image.
A pilot test of the cough recognition camera in an office environment shows that it successfully distinguishes cough events and other events even in a noisy environment. In addition, it can track the location of the person who coughed and count the number of coughs in real time. The performance will be improved further with additional training data obtained from other real environments such as hospitals and classrooms.
Professor Park said, “In a pandemic situation like we are experiencing with COVID-19, a cough detection camera can contribute to the prevention and early detection of epidemics in public places. Especially when applied to a hospital room, the patient's condition can be tracked 24 hours a day and support more accurate diagnoses while reducing the effort of the medical staff."
This study was conducted in collaboration with SM Instruments Inc.
Profile: Yong-Hwa Park, Ph.D.
Associate Professor
yhpark@kaist.ac.kr
http://human.kaist.ac.kr/
Human-Machine Interaction Laboratory (HuMaN Lab.)
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr/en/
Daejeon 34141, Korea
Profile: Gyeong Tae Lee
PhD Candidate
hansaram@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Seong Hu Kim
PhD Candidate
tjdgnkim@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Hyeonuk Nam
PhD Candidate
frednam@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Young-Key Kim
CEO
sales@smins.co.kr
http://en.smins.co.kr/
SM Instruments Inc.
Daejeon 34109, Korea
(END)
2020.08.13 View 19082
Deep Learning-Based Cough Recognition Model Helps Detect the Location of Coughing Sounds in Real Time
The Center for Noise and Vibration Control at KAIST announced that their coughing detection camera recognizes where coughing happens, visualizing the locations. The resulting cough recognition camera can track and record information about the person who coughed, their location, and the number of coughs on a real-time basis.
Professor Yong-Hwa Park from the Department of Mechanical Engineering developed a deep learning-based cough recognition model to classify a coughing sound in real time. The coughing event classification model is combined with a sound camera that visualizes their locations in public places. The research team said they achieved a best test accuracy of 87.4 %.
Professor Park said that it will be useful medical equipment during epidemics in public places such as schools, offices, and restaurants, and to constantly monitor patients’ conditions in a hospital room.
Fever and coughing are the most relevant respiratory disease symptoms, among which fever can be recognized remotely using thermal cameras. This new technology is expected to be very helpful for detecting epidemic transmissions in a non-contact way. The cough event classification model is combined with a sound camera that visualizes the cough event and indicates the location in the video image.
To develop a cough recognition model, a supervised learning was conducted with a convolutional neural network (CNN). The model performs binary classification with an input of a one-second sound profile feature, generating output to be either a cough event or something else.
In the training and evaluation, various datasets were collected from Audioset, DEMAND, ETSI, and TIMIT. Coughing and others sounds were extracted from Audioset, and the rest of the datasets were used as background noises for data augmentation so that this model could be generalized for various background noises in public places.
The dataset was augmented by mixing coughing sounds and other sounds from Audioset and background noises with the ratio of 0.15 to 0.75, then the overall volume was adjusted to 0.25 to 1.0 times to generalize the model for various distances.
The training and evaluation datasets were constructed by dividing the augmented dataset by 9:1, and the test dataset was recorded separately in a real office environment.
In the optimization procedure of the network model, training was conducted with various combinations of five acoustic features including spectrogram, Mel-scaled spectrogram and Mel-frequency cepstrum coefficients with seven optimizers. The performance of each combination was compared with the test dataset. The best test accuracy of 87.4% was achieved with Mel-scaled Spectrogram as the acoustic feature and ASGD as the optimizer.
The trained cough recognition model was combined with a sound camera. The sound camera is composed of a microphone array and a camera module. A beamforming process is applied to a collected set of acoustic data to find out the direction of incoming sound source. The integrated cough recognition model determines whether the sound is cough or not. If it is, the location of cough is visualized as a contour image with a ‘cough’ label at the location of the coughing sound source in a video image.
A pilot test of the cough recognition camera in an office environment shows that it successfully distinguishes cough events and other events even in a noisy environment. In addition, it can track the location of the person who coughed and count the number of coughs in real time. The performance will be improved further with additional training data obtained from other real environments such as hospitals and classrooms.
Professor Park said, “In a pandemic situation like we are experiencing with COVID-19, a cough detection camera can contribute to the prevention and early detection of epidemics in public places. Especially when applied to a hospital room, the patient's condition can be tracked 24 hours a day and support more accurate diagnoses while reducing the effort of the medical staff."
This study was conducted in collaboration with SM Instruments Inc.
Profile: Yong-Hwa Park, Ph.D.
Associate Professor
yhpark@kaist.ac.kr
http://human.kaist.ac.kr/
Human-Machine Interaction Laboratory (HuMaN Lab.)
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr/en/
Daejeon 34141, Korea
Profile: Gyeong Tae Lee
PhD Candidate
hansaram@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Seong Hu Kim
PhD Candidate
tjdgnkim@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Hyeonuk Nam
PhD Candidate
frednam@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Young-Key Kim
CEO
sales@smins.co.kr
http://en.smins.co.kr/
SM Instruments Inc.
Daejeon 34109, Korea
(END)
2020.08.13 View 19082 -
 Sulfur-Containing Polymer Generates High Refractive Index and Transparency
Transparent polymer thin film with refractive index exceeding 1.9 to serve as new platform materials for high-end optical device applications
Researchers reported a novel technology enhancing the high transparency of refractive polymer film via a one-step vapor deposition process. The sulfur-containing polymer (SCP) film produced by Professor Sung Gap Im’s research team at KAIST’s Department of Chemical and Biomolecular Engineering has exhibited excellent environmental stability and chemical resistance, which is highly desirable for its application in long-term optical device applications. The high refractive index exceeding 1.9 while being fully transparent in the entire visible range will help expand the applications of optoelectronic devices.
The refractive index is a ratio of the speed of light in a vacuum to the phase velocity of light in a material, used as a measure of how much the path of light is bent when passing through a material. With the miniaturization of various optical parts used in mobile devices and imaging, demand has been rapidly growing for high refractive index transparent materials that induce more light refraction with a thin film.
As polymers have outstanding physical properties and can be easily processed in various forms, they are widely used in a variety of applications such as plastic eyeglass lenses. However, there have been very few polymers developed so far with a refractive index exceeding 1.75, and existing high refractive index polymers require costly materials and complicated manufacturing processes.
Above all, core technologies for producing such materials have been dominated by Japanese companies, causing long-standing challenges for Korean manufacturers. Securing a stable supply of high-performance, high refractive index materials is crucial for the production of optical devices that are lighter, more affordable, and can be freely manipulated.
The research team successfully manufactured a whole new polymer thin film material with a refractive index exceeding 1.9 and excellent transparency, using just a one-step chemical reaction. The SCP film showed outstanding optical transparency across the entire visible light region, presumably due to the uniformly dispersed, short-segment polysulfide chains, which is a distinct feature unachievable in polymerizations with molten sulfur.
The team focused on the fact that elemental sulfur is easily sublimated to produce a high refractive index polymer by polymerizing the vaporized sulfur with a variety of substances. This method suppresses the formation of overly long S-S chains while achieving outstanding thermal stability in high sulfur concentrations and generating transparent non-crystalline polymers across the entire visible spectrum.
Due to the characteristics of the vapor phase process, the high refractive index thin film can be coated not just on silicon wafers or glass substrates, but on a wide range of textured surfaces as well. We believe this thin film polymer is the first to have achieved an ultrahigh refractive index exceeding 1.9.
Professor Im said, “This high-performance polymer film can be created in a simple one-step manner, which is highly advantageous in the synthesis of SCPs with a high refractive index. This will serve as a platform material for future high-end optical device applications.”
This study, in collaboration with research teams from Seoul National University and Kyung Hee University, was reported in Science Advances. (Title: One-Step Vapor-Phase Synthesis of Transparent High-Refractive Index Sulfur-Containing Polymers)
This research was supported by the Ministry of Science and ICT’s Global Frontier Project (Center for Advanced Soft-Electronics), Leading Research Center Support Program (Wearable Platform Materials Technology Center), and Basic Science Research Program (Advanced Research Project).
2020.08.04 View 11022
Sulfur-Containing Polymer Generates High Refractive Index and Transparency
Transparent polymer thin film with refractive index exceeding 1.9 to serve as new platform materials for high-end optical device applications
Researchers reported a novel technology enhancing the high transparency of refractive polymer film via a one-step vapor deposition process. The sulfur-containing polymer (SCP) film produced by Professor Sung Gap Im’s research team at KAIST’s Department of Chemical and Biomolecular Engineering has exhibited excellent environmental stability and chemical resistance, which is highly desirable for its application in long-term optical device applications. The high refractive index exceeding 1.9 while being fully transparent in the entire visible range will help expand the applications of optoelectronic devices.
The refractive index is a ratio of the speed of light in a vacuum to the phase velocity of light in a material, used as a measure of how much the path of light is bent when passing through a material. With the miniaturization of various optical parts used in mobile devices and imaging, demand has been rapidly growing for high refractive index transparent materials that induce more light refraction with a thin film.
As polymers have outstanding physical properties and can be easily processed in various forms, they are widely used in a variety of applications such as plastic eyeglass lenses. However, there have been very few polymers developed so far with a refractive index exceeding 1.75, and existing high refractive index polymers require costly materials and complicated manufacturing processes.
Above all, core technologies for producing such materials have been dominated by Japanese companies, causing long-standing challenges for Korean manufacturers. Securing a stable supply of high-performance, high refractive index materials is crucial for the production of optical devices that are lighter, more affordable, and can be freely manipulated.
The research team successfully manufactured a whole new polymer thin film material with a refractive index exceeding 1.9 and excellent transparency, using just a one-step chemical reaction. The SCP film showed outstanding optical transparency across the entire visible light region, presumably due to the uniformly dispersed, short-segment polysulfide chains, which is a distinct feature unachievable in polymerizations with molten sulfur.
The team focused on the fact that elemental sulfur is easily sublimated to produce a high refractive index polymer by polymerizing the vaporized sulfur with a variety of substances. This method suppresses the formation of overly long S-S chains while achieving outstanding thermal stability in high sulfur concentrations and generating transparent non-crystalline polymers across the entire visible spectrum.
Due to the characteristics of the vapor phase process, the high refractive index thin film can be coated not just on silicon wafers or glass substrates, but on a wide range of textured surfaces as well. We believe this thin film polymer is the first to have achieved an ultrahigh refractive index exceeding 1.9.
Professor Im said, “This high-performance polymer film can be created in a simple one-step manner, which is highly advantageous in the synthesis of SCPs with a high refractive index. This will serve as a platform material for future high-end optical device applications.”
This study, in collaboration with research teams from Seoul National University and Kyung Hee University, was reported in Science Advances. (Title: One-Step Vapor-Phase Synthesis of Transparent High-Refractive Index Sulfur-Containing Polymers)
This research was supported by the Ministry of Science and ICT’s Global Frontier Project (Center for Advanced Soft-Electronics), Leading Research Center Support Program (Wearable Platform Materials Technology Center), and Basic Science Research Program (Advanced Research Project).
2020.08.04 View 11022 -
 Study Finds Interferon Triggers Inflammation in Severe COVID-19
KAIST medical scientists and their colleagues confirmed that the type I interferon response plays a pivotal role in exacerbating inflammation in severe COVID-19 cases. Severe COVID-19 has been shown to be caused by a hyper-inflammatory response. Particularly, inflammatory cytokines secreted by classical monocytes and macrophages are believed to play a crucial role in the severe progression of COVID-19.
A new single-cell RNA sequencing analysis of more than 59,000 cells from three different patient cohorts provided a detailed look at patients’ immune responses in severe cases of COVID-19. The results suggest that patients with severe cases of COVID-19 experience increased regulation of the type I interferon (IFN-I) inflammation-triggering pathway, a signature that the researchers also observed in patients hospitalized with severe cases of influenza.
Their findings suggest that anti-inflammatory treatment strategies for COVID-19 should also be aimed toward the IFN-I signaling pathway, in addition to targeting inflammatory molecules such as TNF, IL-1, and IL-6, which have been associated with COVID-19.
The research team under Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering sequenced the RNA from a total of 59,572 blood cells obtained from four healthy donors, eight patients with mild or severe COVID-19, and five patients with severe influenza.
By comparison, patients with severe cases of influenza showed increased expression of various IFN-stimulated genes, but did not experience TNF/IL-1 responses as seen in COVID-19 patients. Unlike the flu cohort, patients in the severe COVID-19 cohort exhibited the IFN-I signature concurrently with TNF/IL-1-driven inflammation – a combination also not seen in patients with milder cases of COVID-19.
Their result, along with past mouse studies that highlight how the timing of IFN-I expression is critical to determining the outcome of SARS, support targeting IFN-I as a potential treatment strategy for severe COVID-19.
Professor Shin said, “This research provides insights for designing therapeutic options for COVID-19 by investigating very closely how the immune cells of COVDI-19 patients develop. We will continue to conduct research on novel therapeutic immune mechanisms and target therapeutic anti-inflammatory medication to improve the survival of severe COVID-19 patients.”
This study, conducted in collaboration with Severance Hospital at Yonsei University, Asan Medical Center, and Chungbuk National University, was featured in Science Immunology on July 10. This work was funded by Samsung Science and Technology Foundation and SUHF Fellowship.
-PublicationScience Immunology 10 Jul 2020:Vol. 5, Issue 49, eabd1554DOI: 10.1126/sciimmunol.abd1554
-ProfileProfessorEui-Cheol ShinGraduate School of Medical Science and EngineeringLaboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)euicheols@kaist.ac.krKAIST
2020.07.14 View 9880
Study Finds Interferon Triggers Inflammation in Severe COVID-19
KAIST medical scientists and their colleagues confirmed that the type I interferon response plays a pivotal role in exacerbating inflammation in severe COVID-19 cases. Severe COVID-19 has been shown to be caused by a hyper-inflammatory response. Particularly, inflammatory cytokines secreted by classical monocytes and macrophages are believed to play a crucial role in the severe progression of COVID-19.
A new single-cell RNA sequencing analysis of more than 59,000 cells from three different patient cohorts provided a detailed look at patients’ immune responses in severe cases of COVID-19. The results suggest that patients with severe cases of COVID-19 experience increased regulation of the type I interferon (IFN-I) inflammation-triggering pathway, a signature that the researchers also observed in patients hospitalized with severe cases of influenza.
Their findings suggest that anti-inflammatory treatment strategies for COVID-19 should also be aimed toward the IFN-I signaling pathway, in addition to targeting inflammatory molecules such as TNF, IL-1, and IL-6, which have been associated with COVID-19.
The research team under Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering sequenced the RNA from a total of 59,572 blood cells obtained from four healthy donors, eight patients with mild or severe COVID-19, and five patients with severe influenza.
By comparison, patients with severe cases of influenza showed increased expression of various IFN-stimulated genes, but did not experience TNF/IL-1 responses as seen in COVID-19 patients. Unlike the flu cohort, patients in the severe COVID-19 cohort exhibited the IFN-I signature concurrently with TNF/IL-1-driven inflammation – a combination also not seen in patients with milder cases of COVID-19.
Their result, along with past mouse studies that highlight how the timing of IFN-I expression is critical to determining the outcome of SARS, support targeting IFN-I as a potential treatment strategy for severe COVID-19.
Professor Shin said, “This research provides insights for designing therapeutic options for COVID-19 by investigating very closely how the immune cells of COVDI-19 patients develop. We will continue to conduct research on novel therapeutic immune mechanisms and target therapeutic anti-inflammatory medication to improve the survival of severe COVID-19 patients.”
This study, conducted in collaboration with Severance Hospital at Yonsei University, Asan Medical Center, and Chungbuk National University, was featured in Science Immunology on July 10. This work was funded by Samsung Science and Technology Foundation and SUHF Fellowship.
-PublicationScience Immunology 10 Jul 2020:Vol. 5, Issue 49, eabd1554DOI: 10.1126/sciimmunol.abd1554
-ProfileProfessorEui-Cheol ShinGraduate School of Medical Science and EngineeringLaboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)euicheols@kaist.ac.krKAIST
2020.07.14 View 9880