Ro
-
 Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-Publication
Park, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-Profile
Professor Seongjun Park
Bio and Neural Interfaces Laboratory
Department of Bio and Brain Engineering
KAIST
2020.07.13 View 9399
Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-Publication
Park, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-Profile
Professor Seongjun Park
Bio and Neural Interfaces Laboratory
Department of Bio and Brain Engineering
KAIST
2020.07.13 View 9399 -
 Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11531
Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11531 -
 ‘Mole-bot’ Optimized for Underground and Space Exploration
Biomimetic drilling robot provides new insights into the development of efficient drilling technologies
Mole-bot, a drilling biomimetic robot designed by KAIST, boasts a stout scapula, a waist inclinable on all sides, and powerful forelimbs. Most of all, the powerful torque from the expandable drilling bit mimicking the chiseling ability of a mole’s front teeth highlights the best feature of the drilling robot.
The Mole-bot is expected to be used for space exploration and mining for underground resources such as coalbed methane and Rare Earth Elements (REE), which require highly advanced drilling technologies in complex environments.
The research team, led by Professor Hyun Myung from the School of Electrical Engineering, found inspiration for their drilling bot from two striking features of the African mole-rat and European mole.
“The crushing power of the African mole-rat’s teeth is so powerful that they can dig a hole with 48 times more power than their body weight. We used this characteristic for building the main excavation tool. And its expandable drill is designed not to collide with its forelimbs,” said Professor Myung.
The 25-cm wide and 84-cm long Mole-bot can excavate three times faster with six times higher directional accuracy than conventional models. The Mole-bot weighs 26 kg.
After digging, the robot removes the excavated soil and debris using its forelimbs. This embedded muscle feature, inspired by the European mole’s scapula, converts linear motion into a powerful rotational force. For directional drilling, the robot’s elongated waist changes its direction 360° like living mammals.
For exploring underground environments, the research team developed and applied new sensor systems and algorithms to identify the robot’s position and orientation using graph-based 3D Simultaneous Localization and Mapping (SLAM) technology that matches the Earth’s magnetic field sequence, which enables 3D autonomous navigation underground.
According to Market & Market’s survey, the directional drilling market in 2016 is estimated to be 83.3 billion USD and is expected to grow to 103 billion USD in 2021. The growth of the drilling market, starting with the Shale Revolution, is likely to expand into the future development of space and polar resources. As initiated by Space X recently, more attention for planetary exploration will be on the rise and its related technology and equipment market will also increase.
The Mole-bot is a huge step forward for efficient underground drilling and exploration technologies. Unlike conventional drilling processes that use environmentally unfriendly mud compounds for cleaning debris, Mole-bot can mitigate environmental destruction. The researchers said their system saves on cost and labor and does not require additional pipelines or other ancillary equipment.
“We look forward to a more efficient resource exploration with this type of drilling robot. We also hope Mole-bot will have a very positive impact on the robotics market in terms of its extensive application spectra and economic feasibility,” said Professor Myung.
This research, made in collaboration with Professor Jung-Wuk Hong and Professor Tae-Hyuk Kwon’s team in the Department of Civil and Environmental Engineering for robot structure analysis and geotechnical experiments, was supported by the Ministry of Trade, Industry and Energy’s Industrial Technology Innovation Project.
Profile
Professor Hyun Myung
Urban Robotics Lab
http://urobot.kaist.ac.kr/
School of Electrical Engineering
KAIST
2020.06.05 View 12874
‘Mole-bot’ Optimized for Underground and Space Exploration
Biomimetic drilling robot provides new insights into the development of efficient drilling technologies
Mole-bot, a drilling biomimetic robot designed by KAIST, boasts a stout scapula, a waist inclinable on all sides, and powerful forelimbs. Most of all, the powerful torque from the expandable drilling bit mimicking the chiseling ability of a mole’s front teeth highlights the best feature of the drilling robot.
The Mole-bot is expected to be used for space exploration and mining for underground resources such as coalbed methane and Rare Earth Elements (REE), which require highly advanced drilling technologies in complex environments.
The research team, led by Professor Hyun Myung from the School of Electrical Engineering, found inspiration for their drilling bot from two striking features of the African mole-rat and European mole.
“The crushing power of the African mole-rat’s teeth is so powerful that they can dig a hole with 48 times more power than their body weight. We used this characteristic for building the main excavation tool. And its expandable drill is designed not to collide with its forelimbs,” said Professor Myung.
The 25-cm wide and 84-cm long Mole-bot can excavate three times faster with six times higher directional accuracy than conventional models. The Mole-bot weighs 26 kg.
After digging, the robot removes the excavated soil and debris using its forelimbs. This embedded muscle feature, inspired by the European mole’s scapula, converts linear motion into a powerful rotational force. For directional drilling, the robot’s elongated waist changes its direction 360° like living mammals.
For exploring underground environments, the research team developed and applied new sensor systems and algorithms to identify the robot’s position and orientation using graph-based 3D Simultaneous Localization and Mapping (SLAM) technology that matches the Earth’s magnetic field sequence, which enables 3D autonomous navigation underground.
According to Market & Market’s survey, the directional drilling market in 2016 is estimated to be 83.3 billion USD and is expected to grow to 103 billion USD in 2021. The growth of the drilling market, starting with the Shale Revolution, is likely to expand into the future development of space and polar resources. As initiated by Space X recently, more attention for planetary exploration will be on the rise and its related technology and equipment market will also increase.
The Mole-bot is a huge step forward for efficient underground drilling and exploration technologies. Unlike conventional drilling processes that use environmentally unfriendly mud compounds for cleaning debris, Mole-bot can mitigate environmental destruction. The researchers said their system saves on cost and labor and does not require additional pipelines or other ancillary equipment.
“We look forward to a more efficient resource exploration with this type of drilling robot. We also hope Mole-bot will have a very positive impact on the robotics market in terms of its extensive application spectra and economic feasibility,” said Professor Myung.
This research, made in collaboration with Professor Jung-Wuk Hong and Professor Tae-Hyuk Kwon’s team in the Department of Civil and Environmental Engineering for robot structure analysis and geotechnical experiments, was supported by the Ministry of Trade, Industry and Energy’s Industrial Technology Innovation Project.
Profile
Professor Hyun Myung
Urban Robotics Lab
http://urobot.kaist.ac.kr/
School of Electrical Engineering
KAIST
2020.06.05 View 12874 -
 Universal Virus Detection Platform to Expedite Viral Diagnosis
Reactive polymer-based tester pre-screens dsRNAs of a wide range of viruses without their genome sequences
The prompt, precise, and massive detection of a virus is the key to combat infectious diseases such as Covid-19. A new viral diagnostic strategy using reactive polymer-grafted, double-stranded RNAs will serve as a pre-screening tester for a wide range of viruses with enhanced sensitivity.
Currently, the most widely using viral detection methodology is polymerase chain reaction (PCR) diagnosis, which amplifies and detects a piece of the viral genome. Prior knowledge of the relevant primer nucleic acids of the virus is quintessential for this test.
The detection platform developed by KAIST researchers identifies viral activities without amplifying specific nucleic acid targets. The research team, co-led by Professor Sheng Li and Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering, constructed a universal virus detection platform by utilizing the distinct features of the PPFPA-grafted surface and double-stranded RNAs.
The key principle of this platform is utilizing the distinct feature of reactive polymer-grafted surfaces, which serve as a versatile platform for the immobilization of functional molecules. These activated surfaces can be used in a wide range of applications including separation, delivery, and detection. As long double-stranded RNAs are common byproducts of viral transcription and replication, these PPFPA-grafted surfaces can detect the presence of different kinds of viruses without prior knowledge of their genomic sequences.
“We employed the PPFPA-grafted silicon surface to develop a universal virus detection platform by immobilizing antibodies that recognize double-stranded RNAs,” said Professor Kim.
To increase detection sensitivity, the research team devised two-step detection process analogues to sandwich enzyme-linked immunosorbent assay where the bound double-stranded RNAs are then visualized using fluorophore-tagged antibodies that also recognize the RNAs’ double-stranded secondary structure.
By utilizing the developed platform, long double-stranded RNAs can be detected and visualized from an RNA mixture as well as from total cell lysates, which contain a mixture of various abundant contaminants such as DNAs and proteins.
The research team successfully detected elevated levels of hepatitis C and A viruses with this tool.
“This new technology allows us to take on virus detection from a new perspective. By targeting a common biomarker, viral double-stranded RNAs, we can develop a pre-screening platform that can quickly differentiate infected populations from non-infected ones,” said Professor Li.
“This detection platform provides new perspectives for diagnosing infectious diseases. This will provide fast and accurate diagnoses for an infected population and prevent the influx of massive outbreaks,” said Professor Kim.
This work is featured in Biomacromolecules. This work was supported by the Agency for Defense Development (Grant UD170039ID), the Ministry of Science and ICT (NRF-2017R1D1A1B03034660, NRF-2019R1C1C1006672), and the KAIST Future Systems Healthcare Project from the Ministry of Science and ICT (KAISTHEALTHCARE42).
Profile:-Professor Yoosik KimDepartment of Chemical and Biomolecular Engineeringhttps://qcbio.kaist.ac.kr
KAIST-Professor Sheng LiDepartment of Chemical and Biomolecular Engineeringhttps://bcpolymer.kaist.ac.kr
KAIST
Publication:Ku et al., 2020. Reactive Polymer Targeting dsRNA as Universal Virus Detection Platform with Enhanced Sensitivity. Biomacromolecules (https://doi.org/10.1021/acs.biomac.0c00379).
2020.06.01 View 20801
Universal Virus Detection Platform to Expedite Viral Diagnosis
Reactive polymer-based tester pre-screens dsRNAs of a wide range of viruses without their genome sequences
The prompt, precise, and massive detection of a virus is the key to combat infectious diseases such as Covid-19. A new viral diagnostic strategy using reactive polymer-grafted, double-stranded RNAs will serve as a pre-screening tester for a wide range of viruses with enhanced sensitivity.
Currently, the most widely using viral detection methodology is polymerase chain reaction (PCR) diagnosis, which amplifies and detects a piece of the viral genome. Prior knowledge of the relevant primer nucleic acids of the virus is quintessential for this test.
The detection platform developed by KAIST researchers identifies viral activities without amplifying specific nucleic acid targets. The research team, co-led by Professor Sheng Li and Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering, constructed a universal virus detection platform by utilizing the distinct features of the PPFPA-grafted surface and double-stranded RNAs.
The key principle of this platform is utilizing the distinct feature of reactive polymer-grafted surfaces, which serve as a versatile platform for the immobilization of functional molecules. These activated surfaces can be used in a wide range of applications including separation, delivery, and detection. As long double-stranded RNAs are common byproducts of viral transcription and replication, these PPFPA-grafted surfaces can detect the presence of different kinds of viruses without prior knowledge of their genomic sequences.
“We employed the PPFPA-grafted silicon surface to develop a universal virus detection platform by immobilizing antibodies that recognize double-stranded RNAs,” said Professor Kim.
To increase detection sensitivity, the research team devised two-step detection process analogues to sandwich enzyme-linked immunosorbent assay where the bound double-stranded RNAs are then visualized using fluorophore-tagged antibodies that also recognize the RNAs’ double-stranded secondary structure.
By utilizing the developed platform, long double-stranded RNAs can be detected and visualized from an RNA mixture as well as from total cell lysates, which contain a mixture of various abundant contaminants such as DNAs and proteins.
The research team successfully detected elevated levels of hepatitis C and A viruses with this tool.
“This new technology allows us to take on virus detection from a new perspective. By targeting a common biomarker, viral double-stranded RNAs, we can develop a pre-screening platform that can quickly differentiate infected populations from non-infected ones,” said Professor Li.
“This detection platform provides new perspectives for diagnosing infectious diseases. This will provide fast and accurate diagnoses for an infected population and prevent the influx of massive outbreaks,” said Professor Kim.
This work is featured in Biomacromolecules. This work was supported by the Agency for Defense Development (Grant UD170039ID), the Ministry of Science and ICT (NRF-2017R1D1A1B03034660, NRF-2019R1C1C1006672), and the KAIST Future Systems Healthcare Project from the Ministry of Science and ICT (KAISTHEALTHCARE42).
Profile:-Professor Yoosik KimDepartment of Chemical and Biomolecular Engineeringhttps://qcbio.kaist.ac.kr
KAIST-Professor Sheng LiDepartment of Chemical and Biomolecular Engineeringhttps://bcpolymer.kaist.ac.kr
KAIST
Publication:Ku et al., 2020. Reactive Polymer Targeting dsRNA as Universal Virus Detection Platform with Enhanced Sensitivity. Biomacromolecules (https://doi.org/10.1021/acs.biomac.0c00379).
2020.06.01 View 20801 -
 Professor Tek-jin Nam Elected to DSR Int’l Advisory Council
Professor Tek-jin Nam from the Department of Industrial Design was elected to serve on the first International Advisory Council (IAC) of the Design Research Society (DRS).
The DRS, an academic society in the field of design research, was founded in the UK in 1966 with the mission of developing and promoting design research. The IAC is newly established under the new DRS governance structure, and its members are selected from distinguished design researchers recommended by DRS members around the globe.
The new IAC members will carry out various activities offered by the DRS, which include innovating design research, strengthening the design researchers’ network and developing policies to nurture new researchers.
2020.05.22 View 6935
Professor Tek-jin Nam Elected to DSR Int’l Advisory Council
Professor Tek-jin Nam from the Department of Industrial Design was elected to serve on the first International Advisory Council (IAC) of the Design Research Society (DRS).
The DRS, an academic society in the field of design research, was founded in the UK in 1966 with the mission of developing and promoting design research. The IAC is newly established under the new DRS governance structure, and its members are selected from distinguished design researchers recommended by DRS members around the globe.
The new IAC members will carry out various activities offered by the DRS, which include innovating design research, strengthening the design researchers’ network and developing policies to nurture new researchers.
2020.05.22 View 6935 -
 Algorithm Identifies Optimal Pairs for Composing Metal-Organic Frameworks
The integration of metal-organic frameworks (MOFs) and other metal nanoparticles has increasingly led to the creation of new multifunctional materials. Many researchers have integrated MOFs with other classes of materials to produce new structures with synergetic properties.
Despite there being over 70,000 collections of synthesized MOFs that can be used as building blocks, the precise nature of the interaction and the bonding at the interface between the two materials still remains unknown. The question is how to sort out the right matching pairs out of 70,000 MOFs.
An algorithmic study published in Nature Communications by a KAIST research team presents a clue for finding the perfect pairs. The team, led by Professor Ji-Han Kim from the Department of Chemical and Biomolecular Engineering, developed a joint computational and experimental approach to rationally design MOF@MOFs, a composite of MOFs where an MOF is grown on a different MOF.
Professor Kim’s team, in collaboration with UNIST, noted that the metal node of one MOF can coordinately bond with the linker of a different MOF and the precisely matched interface configurations at atomic and molecular levels can enhance the likelihood of synthesizing MOF@MOFs.
They screened thousands of MOFs and identified optimal MOF pairs that can seamlessly connect to one another by taking advantage of the fact that the metal node of one MOF can form coordination bonds with the linkers of the second MOF. Six pairs predicted from the computational algorithm successfully grew into single crystals.
This computational workflow can readily extend into other classes of materials and can lead to the rapid exploration of the composite MOFs arena for accelerated materials development. Even more, the workflow can enhance the likelihood of synthesizing MOF@MOFs in the form of large single crystals, and thereby demonstrated the utility of rationally designing the MOF@MOFs.
This study is the first algorithm for predicting the synthesis of composite MOFs, to the best of their knowledge. Professor Kim said, “The number of predicted pairs can increase even more with the more general 2D lattice matching, and it is worth investigating in the future.”
This study was supported by Samsung Research Funding & Incubation Center of Samsung Electronics.
(Figure: An example of a rationally synthesized MOF@MOFs (cubic HKUST-1@MOF-5 ))
2019.08.30 View 18474
Algorithm Identifies Optimal Pairs for Composing Metal-Organic Frameworks
The integration of metal-organic frameworks (MOFs) and other metal nanoparticles has increasingly led to the creation of new multifunctional materials. Many researchers have integrated MOFs with other classes of materials to produce new structures with synergetic properties.
Despite there being over 70,000 collections of synthesized MOFs that can be used as building blocks, the precise nature of the interaction and the bonding at the interface between the two materials still remains unknown. The question is how to sort out the right matching pairs out of 70,000 MOFs.
An algorithmic study published in Nature Communications by a KAIST research team presents a clue for finding the perfect pairs. The team, led by Professor Ji-Han Kim from the Department of Chemical and Biomolecular Engineering, developed a joint computational and experimental approach to rationally design MOF@MOFs, a composite of MOFs where an MOF is grown on a different MOF.
Professor Kim’s team, in collaboration with UNIST, noted that the metal node of one MOF can coordinately bond with the linker of a different MOF and the precisely matched interface configurations at atomic and molecular levels can enhance the likelihood of synthesizing MOF@MOFs.
They screened thousands of MOFs and identified optimal MOF pairs that can seamlessly connect to one another by taking advantage of the fact that the metal node of one MOF can form coordination bonds with the linkers of the second MOF. Six pairs predicted from the computational algorithm successfully grew into single crystals.
This computational workflow can readily extend into other classes of materials and can lead to the rapid exploration of the composite MOFs arena for accelerated materials development. Even more, the workflow can enhance the likelihood of synthesizing MOF@MOFs in the form of large single crystals, and thereby demonstrated the utility of rationally designing the MOF@MOFs.
This study is the first algorithm for predicting the synthesis of composite MOFs, to the best of their knowledge. Professor Kim said, “The number of predicted pairs can increase even more with the more general 2D lattice matching, and it is worth investigating in the future.”
This study was supported by Samsung Research Funding & Incubation Center of Samsung Electronics.
(Figure: An example of a rationally synthesized MOF@MOFs (cubic HKUST-1@MOF-5 ))
2019.08.30 View 18474 -
 Distinguished Professor Sukbok Chang Donates His Prize Money
The honoree of the 2019 Korea Best Scientist and Technologist Award, Distinguished Professor Sukbok Chang donated his prize money of one hundred million KRW to the Chemistry Department Scholarship Fund and the Lyu Keun-Chul Sports Complex Management Fund during a donation ceremony last week.
Professor Chang won the award last month in recognition of his pioneering achievements and lifetime contributions to the development of carbon-hydrogen activation strategies, especially for carbon-carbon, carbon-nitrogen, and carbon-oxygen formations. Professor Chang, a world renowned chemist, has been recognized for his highly selective catalytic systems, allowing the controlled defunctionalization of bio-derived platform substrates under mild conditions and opening a new avenue for the utilization of biomass-derived platform chemicals.
“All my achievements are the results of my students’ hard work and dedication. I feel very fortunate to have such talented team members. I want to express my sincere gratitude for such a great research environment that we have worked together in so far,” said Professor Chang at the ceremony.
KAIST President Sung-Chul Shin said, “Not only will Professor Chang’s donation make a significant contribution to the Department of Chemistry, but also to the improvement of the Lyu Keun-Chul Sports Complex’s management, which directly links to the health and welfare of the KAIST community.”
Professor Chang currently holds the position of distinguished professor at KAIST and director of the Center for Catalytic Hydrocarbon Functionalizations in the Institute for Basic Science (IBS). He previously received the Kyung-Ahm Academic Award in 2013 and the Korea Toray Science Award in 2018. All these prize money also went to the school.
(END)
2019.08.26 View 9785
Distinguished Professor Sukbok Chang Donates His Prize Money
The honoree of the 2019 Korea Best Scientist and Technologist Award, Distinguished Professor Sukbok Chang donated his prize money of one hundred million KRW to the Chemistry Department Scholarship Fund and the Lyu Keun-Chul Sports Complex Management Fund during a donation ceremony last week.
Professor Chang won the award last month in recognition of his pioneering achievements and lifetime contributions to the development of carbon-hydrogen activation strategies, especially for carbon-carbon, carbon-nitrogen, and carbon-oxygen formations. Professor Chang, a world renowned chemist, has been recognized for his highly selective catalytic systems, allowing the controlled defunctionalization of bio-derived platform substrates under mild conditions and opening a new avenue for the utilization of biomass-derived platform chemicals.
“All my achievements are the results of my students’ hard work and dedication. I feel very fortunate to have such talented team members. I want to express my sincere gratitude for such a great research environment that we have worked together in so far,” said Professor Chang at the ceremony.
KAIST President Sung-Chul Shin said, “Not only will Professor Chang’s donation make a significant contribution to the Department of Chemistry, but also to the improvement of the Lyu Keun-Chul Sports Complex’s management, which directly links to the health and welfare of the KAIST community.”
Professor Chang currently holds the position of distinguished professor at KAIST and director of the Center for Catalytic Hydrocarbon Functionalizations in the Institute for Basic Science (IBS). He previously received the Kyung-Ahm Academic Award in 2013 and the Korea Toray Science Award in 2018. All these prize money also went to the school.
(END)
2019.08.26 View 9785 -
 Deciphering Brain Somatic Mutations Associated with Alzheimer's Disease
Researchers have found a potential link between non-inherited somatic mutations in the brain and the progression of Alzheimer’s disease
Researchers have identified somatic mutations in the brain that could contribute to the development of Alzheimer’s disease (AD). Their findings were published in the journal Nature Communications last week.
Decades worth of research has identified inherited mutations that lead to early-onset familial AD. Inherited mutations, however, are behind at most half the cases of late onset sporadic AD, in which there is no family history of the disease. But the genetic factors causing the other half of these sporadic cases have been unclear.
Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering and colleagues analysed the DNA present in post-mortem hippocampal formations and in blood samples from people aged 70 to 96 with AD and age-matched controls. They specifically looked for non-inherited somatic mutations in their brains using high-depth whole exome sequencing.
The team developed a bioinformatics pipeline that enabled them to detect low-level brain somatic single nucleotide variations (SNVs) – mutations that involve the substitution of a single nucleotide with another nucleotide. Brain somatic SNVs have been reported on and accumulate throughout our lives and can sometimes be associated with a range of neurological diseases.
The number of somatic SNVs did not differ between individuals with AD and non-demented controls. Interestingly, somatic SNVs in AD brains arise about 4.8 times more slowly than in blood. When the team performed gene-set enrichment tests, 26.9 percent of the AD brain samples had pathogenic brain somatic SNVs known to be linked to hyperphosphorylation of tau proteins, which is one of major hallmarks of AD.
Then, they pinpointed a pathogenic SNV in the PIN1 gene, a cis/trans isomerase that balances phosphorylation in tau proteins, found in one AD patient’s brain. They found the mutation was 4.9 time more abundant in AT8-positive – a marker for hyper-phosphorylated tau proteins– neurons in the entorhinal cortex than the bulk hippocampal tissue. Furthermore, in a series of functional assays, they observed the mutation causing a loss of function in PIN1 and such haploinsufficiency increased the phosphorylation and aggregation of tau proteins.
“Our study provides new insights into the molecular genetic factors behind Alzheimer’s disease and other neurodegenerative diseases potentially linked to somatic mutations in the brain,” said Professor Lee.
The team is planning to expand their study to a larger cohort in order to establish stronger links between these brain somatic mutations and the pathogenesis of Alzheimer’s disease.
(Figure 1. Bioinformatic pipeline for detecting low-level brain somatic mutations in AD and non-AD.)
(Figure 2. Pathogenic brain somatic mutations associated with tau phosphorylation are significantly enriched in AD brains.)
(Figure 3. A pathogenic brain somatic mutation in PIN1 (c. 477 C>T) is a loss-of-function and related functional assays show its haploinsufficiency increases phosphorylation and aggregation of tau.)
2019.07.19 View 37839
Deciphering Brain Somatic Mutations Associated with Alzheimer's Disease
Researchers have found a potential link between non-inherited somatic mutations in the brain and the progression of Alzheimer’s disease
Researchers have identified somatic mutations in the brain that could contribute to the development of Alzheimer’s disease (AD). Their findings were published in the journal Nature Communications last week.
Decades worth of research has identified inherited mutations that lead to early-onset familial AD. Inherited mutations, however, are behind at most half the cases of late onset sporadic AD, in which there is no family history of the disease. But the genetic factors causing the other half of these sporadic cases have been unclear.
Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering and colleagues analysed the DNA present in post-mortem hippocampal formations and in blood samples from people aged 70 to 96 with AD and age-matched controls. They specifically looked for non-inherited somatic mutations in their brains using high-depth whole exome sequencing.
The team developed a bioinformatics pipeline that enabled them to detect low-level brain somatic single nucleotide variations (SNVs) – mutations that involve the substitution of a single nucleotide with another nucleotide. Brain somatic SNVs have been reported on and accumulate throughout our lives and can sometimes be associated with a range of neurological diseases.
The number of somatic SNVs did not differ between individuals with AD and non-demented controls. Interestingly, somatic SNVs in AD brains arise about 4.8 times more slowly than in blood. When the team performed gene-set enrichment tests, 26.9 percent of the AD brain samples had pathogenic brain somatic SNVs known to be linked to hyperphosphorylation of tau proteins, which is one of major hallmarks of AD.
Then, they pinpointed a pathogenic SNV in the PIN1 gene, a cis/trans isomerase that balances phosphorylation in tau proteins, found in one AD patient’s brain. They found the mutation was 4.9 time more abundant in AT8-positive – a marker for hyper-phosphorylated tau proteins– neurons in the entorhinal cortex than the bulk hippocampal tissue. Furthermore, in a series of functional assays, they observed the mutation causing a loss of function in PIN1 and such haploinsufficiency increased the phosphorylation and aggregation of tau proteins.
“Our study provides new insights into the molecular genetic factors behind Alzheimer’s disease and other neurodegenerative diseases potentially linked to somatic mutations in the brain,” said Professor Lee.
The team is planning to expand their study to a larger cohort in order to establish stronger links between these brain somatic mutations and the pathogenesis of Alzheimer’s disease.
(Figure 1. Bioinformatic pipeline for detecting low-level brain somatic mutations in AD and non-AD.)
(Figure 2. Pathogenic brain somatic mutations associated with tau phosphorylation are significantly enriched in AD brains.)
(Figure 3. A pathogenic brain somatic mutation in PIN1 (c. 477 C>T) is a loss-of-function and related functional assays show its haploinsufficiency increases phosphorylation and aggregation of tau.)
2019.07.19 View 37839 -
 High-Performance Sodium Ion Batteries Using Copper Sulfide
(Prof.Yuk and his two PhD candidates Parks)
Researchers presented a new strategy for extending sodium ion batteries’ cyclability using copper sulfide as the electrode material. This strategy has led to high-performance conversion reactions and is expected to advance the commercialization of sodium ion batteries as they emerge as an alternative to lithium ion batteries.
Professor Jong Min Yuk’s team confirmed the stable sodium storage mechanism using copper sulfide, a superior electrode material that is pulverization-tolerant and induces capacity recovery. Their findings suggest that when employing copper sulfide, sodium ion batteries will have a lifetime of more than five years with one charge per a day. Even better, copper sulfide, composed of abundant natural materials such as copper and sulfur, has better cost competitiveness than lithium ion batteries, which use lithium and cobalt.
Intercalation-type materials such as graphite, which serve as commercialized anode materials in lithium ion batteries, have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring up large volume expansions and abrupt crystallographic changes, which lead to severe capacity degradation.
The team confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played key roles in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Most of conversion and alloying reactions type battery materials usually experience severe capacity degradations due to having completely different crystal structures and large volume expansion before and after the reactions. However, copper sulfides underwent a gradual crystallographic change to make the semi-coherent interfaces, which eventually prevented the pulverization of particles. Based on this unique mechanism, the team confirmed that copper sulfide exhibits a high capacity and high cycling stability regardless of its size and morphology.
Professor Yuk said, “Sodium ion batteries employing copper sulfide can advance sodium ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue”
This study was posted in Advanced Science on April 26 online and selected as the inside back cover for June issue.
(Figure: Schematic model demonstrating grain boundaries and phase interfaces formations.)
2019.07.15 View 29500
High-Performance Sodium Ion Batteries Using Copper Sulfide
(Prof.Yuk and his two PhD candidates Parks)
Researchers presented a new strategy for extending sodium ion batteries’ cyclability using copper sulfide as the electrode material. This strategy has led to high-performance conversion reactions and is expected to advance the commercialization of sodium ion batteries as they emerge as an alternative to lithium ion batteries.
Professor Jong Min Yuk’s team confirmed the stable sodium storage mechanism using copper sulfide, a superior electrode material that is pulverization-tolerant and induces capacity recovery. Their findings suggest that when employing copper sulfide, sodium ion batteries will have a lifetime of more than five years with one charge per a day. Even better, copper sulfide, composed of abundant natural materials such as copper and sulfur, has better cost competitiveness than lithium ion batteries, which use lithium and cobalt.
Intercalation-type materials such as graphite, which serve as commercialized anode materials in lithium ion batteries, have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring up large volume expansions and abrupt crystallographic changes, which lead to severe capacity degradation.
The team confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played key roles in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Most of conversion and alloying reactions type battery materials usually experience severe capacity degradations due to having completely different crystal structures and large volume expansion before and after the reactions. However, copper sulfides underwent a gradual crystallographic change to make the semi-coherent interfaces, which eventually prevented the pulverization of particles. Based on this unique mechanism, the team confirmed that copper sulfide exhibits a high capacity and high cycling stability regardless of its size and morphology.
Professor Yuk said, “Sodium ion batteries employing copper sulfide can advance sodium ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue”
This study was posted in Advanced Science on April 26 online and selected as the inside back cover for June issue.
(Figure: Schematic model demonstrating grain boundaries and phase interfaces formations.)
2019.07.15 View 29500 -
 KAIST Identifies the Cause of Sepsis-induced Lung Injury
(Professor Pilhan Kim from the Graduate School of Medical Science and Engineering)
A KAIST research team succeeded in visualizing pulmonary microcirculation and circulating cells in vivo with a custom-built 3D intravital lung microscopic imaging system. They found a type of leukocyte called neutrophils aggregate inside the capillaries during sepsis-induced acute lung injury (ALI), leading to disturbances and dead space in blood microcirculation.
According to the researchers, this phenomenon is responsible for tissue hypoxia causing lung damage in the sepsis model, and mitigating neutrophils improves microcirculation as well as hypoxia.
The lungs are responsible for exchanging oxygen with carbon dioxide gases during the breathing process, providing an essential function for sustaining life. This gas exchange occurs in the alveoli, each surrounded by many capillaries containing the circulating red blood cells.
Researchers have been making efforts to observe microcirculation in alveoli, but it has been technically challenging to capture high-resolution images of capillaries and red blood cells inside the lungs that are in constant breathing motion.
Professor Pilhan Kim from the Graduate School of Medical Science and Engineering and his team developed an ultra-fast laser scanning confocal microscope and an imaging chamber that could minimize the movement of a lung while preserving its respiratory state. They used this technology to successfully capture red blood cell circulation inside the capillaries of animal models with sepsis.
During the process, they found that hypoxia was induced by the increase of dead space inside the lungs of a sepsis model, a space where red blood cells do not circulate. This phenomenon is due to the neutrophils aggregating and trapping inside the capillaries and the arterioles. It was also shown that trapped neutrophils damage the lung tissue in the sepsis model by inhibiting microcirculation as well as releasing reactive oxygen species.
Further studies showed that the aggregated neutrophils inside pulmonary vessels exhibit a higher expression of the Mac-1 receptor (CD11b/CD18), which is a receptor involved in intercellular adhesion, compared to the neutrophils that normally circulate. Additionally, they confirmed that Mac-1 inhibitors can improve inhibited microcirculation, ameliorate hypoxia, while reducing pulmonary edema in the sepsis model.
Their high-resolution 3D intravital microscope technology allows the real-time imaging of living cells inside the lungs. This work is expected to be used in research on various lung diseases, including sepsis.
The research team’s pulmonary circulation imaging and precise analytical techniques will be used as the base technology for developing new diagnostic technologies, evaluating new therapeutic agents for various diseases related to microcirculation.
Professor Kim said, “In the ALI model, the inhibition of pulmonary microcirculation occurs due to neutrophils. By controlling this effect and improving microcirculation, it is possible to eliminate hypoxia and pulmonary edema – a new, effective strategy for treating patients with sepsis.”
Their 3D intravital microscope technology was commercialized through IVIM Technology, Inc., which is a faculty startup at KAIST. They released an all-in-one intravital microscope model called ‘IVM-CM’ and ‘IVM-C’. This next-generation imaging equipment for basic biomedical research on the complex pathophysiology of various human diseases will play a crucial role in the future global bio-health market.
This research, led by Dr. Inwon Park from the Department of Emergency Medicine at Seoul National University Bundang Hospital and formally the Graduate School of Medical Science and Engineering at KAIST, was published in the European Respiratory Journal (2019, 53:1800736) on March 28, 2019.
Figure 1. Custom-built high-speed real-time intravital microscope platform
Figure 2. Illustrative schematic and photo of a 3D intravital lung microscopic imaging system
Figure 3. Aggregation of neutrophils and consequent flow disturbance in pulmonary arteriole in sepsis-induced lung injury
2019.05.07 View 45476
KAIST Identifies the Cause of Sepsis-induced Lung Injury
(Professor Pilhan Kim from the Graduate School of Medical Science and Engineering)
A KAIST research team succeeded in visualizing pulmonary microcirculation and circulating cells in vivo with a custom-built 3D intravital lung microscopic imaging system. They found a type of leukocyte called neutrophils aggregate inside the capillaries during sepsis-induced acute lung injury (ALI), leading to disturbances and dead space in blood microcirculation.
According to the researchers, this phenomenon is responsible for tissue hypoxia causing lung damage in the sepsis model, and mitigating neutrophils improves microcirculation as well as hypoxia.
The lungs are responsible for exchanging oxygen with carbon dioxide gases during the breathing process, providing an essential function for sustaining life. This gas exchange occurs in the alveoli, each surrounded by many capillaries containing the circulating red blood cells.
Researchers have been making efforts to observe microcirculation in alveoli, but it has been technically challenging to capture high-resolution images of capillaries and red blood cells inside the lungs that are in constant breathing motion.
Professor Pilhan Kim from the Graduate School of Medical Science and Engineering and his team developed an ultra-fast laser scanning confocal microscope and an imaging chamber that could minimize the movement of a lung while preserving its respiratory state. They used this technology to successfully capture red blood cell circulation inside the capillaries of animal models with sepsis.
During the process, they found that hypoxia was induced by the increase of dead space inside the lungs of a sepsis model, a space where red blood cells do not circulate. This phenomenon is due to the neutrophils aggregating and trapping inside the capillaries and the arterioles. It was also shown that trapped neutrophils damage the lung tissue in the sepsis model by inhibiting microcirculation as well as releasing reactive oxygen species.
Further studies showed that the aggregated neutrophils inside pulmonary vessels exhibit a higher expression of the Mac-1 receptor (CD11b/CD18), which is a receptor involved in intercellular adhesion, compared to the neutrophils that normally circulate. Additionally, they confirmed that Mac-1 inhibitors can improve inhibited microcirculation, ameliorate hypoxia, while reducing pulmonary edema in the sepsis model.
Their high-resolution 3D intravital microscope technology allows the real-time imaging of living cells inside the lungs. This work is expected to be used in research on various lung diseases, including sepsis.
The research team’s pulmonary circulation imaging and precise analytical techniques will be used as the base technology for developing new diagnostic technologies, evaluating new therapeutic agents for various diseases related to microcirculation.
Professor Kim said, “In the ALI model, the inhibition of pulmonary microcirculation occurs due to neutrophils. By controlling this effect and improving microcirculation, it is possible to eliminate hypoxia and pulmonary edema – a new, effective strategy for treating patients with sepsis.”
Their 3D intravital microscope technology was commercialized through IVIM Technology, Inc., which is a faculty startup at KAIST. They released an all-in-one intravital microscope model called ‘IVM-CM’ and ‘IVM-C’. This next-generation imaging equipment for basic biomedical research on the complex pathophysiology of various human diseases will play a crucial role in the future global bio-health market.
This research, led by Dr. Inwon Park from the Department of Emergency Medicine at Seoul National University Bundang Hospital and formally the Graduate School of Medical Science and Engineering at KAIST, was published in the European Respiratory Journal (2019, 53:1800736) on March 28, 2019.
Figure 1. Custom-built high-speed real-time intravital microscope platform
Figure 2. Illustrative schematic and photo of a 3D intravital lung microscopic imaging system
Figure 3. Aggregation of neutrophils and consequent flow disturbance in pulmonary arteriole in sepsis-induced lung injury
2019.05.07 View 45476 -
 Wafer-Scale Multilayer Fabrication of Silk Fibroin-Based Microelectronics
A KAIST research team developed a novel fabrication method for the multilayer processing of silk-based microelectronics. This technology for creating a biodegradable silk fibroin film allows microfabrication with polymer or metal structures manufactured from photolithography. It can be a key technology in the implementation of silk fibroin-based biodegradable electronic devices or localized drug delivery through silk fibroin patterns.
Silk fibroins are biocompatible, biodegradable, transparent, and flexible, which makes them excellent candidates for implantable biomedical devices, and they have also been used as biodegradable films and functional microstructures in biomedical applications. However, conventional microfabrication processes require strong etching solutions and solvents to modify the structure of silk fibroins.
To prevent the silk fibroin from being damaged during the process, Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team came up with a novel process, named aluminum hard mask on silk fibroin (AMoS), which is capable of micropatterning multiple layers composed of both fibroin and inorganic materials, such as metal and dielectrics with high-precision microscale alignment. The AMoS process can make silk fibroin patterns on devices, or make patterns on silk fibroin thin films with other materials by using photolithography, which is a core technology in the current microfabrication process.
The team successfully cultured primary neurons on the processed silk fibroin micro-patterns, and confirmed that silk fibroin has excellent biocompatibility before and after the fabrication process and that it also can be applied to implanted biological devices.
Through this technology, the team realized the multilayer micropatterning of fibroin films on a silk fibroin substrate and fabricated a biodegradable microelectric circuit consisting of resistors and silk fibroin dielectric capacitors in a silicon wafer with large areas.
They also used this technology to position the micro-pattern of the silk fibroin thin film closer to the flexible polymer-based brain electrode, and confirmed the dye molecules mounted on the silk fibroin were transferred successfully from the micropatterns.
Professor Lee said, “This technology facilitates wafer-scale, large-area processing of sensitive materials. We expect it to be applied to a wide range of biomedical devices in the future. Using the silk fibroin with micro-patterned brain electrodes can open up many new possibilities in research on brain circuits by mounting drugs that restrict or promote brain cell activities.”
This research, in collaboration with Dr. Nakwon Choi from KIST and led by PhD candidate Geon Kook, was published in ACS AMI (10.1021/acsami.8b13170) on January 16, 2019.
Figure 1. The cover page of ACS AMI
Figure 2. Fibroin microstructures and metal patterns on a fibroin produced by using the AMoS mask.
Figure 3. Biocompatibility assessment of the AMoS Process. Top: Schematics image of a) fibroin-coated silicon b) fibroin-pattered silicon and c) gold-patterned fibroin. Bottom: Representative confocal microscopy images of live (green) and dead (red) primary cortical neurons cultured on the substrates.
2019.03.15 View 23671
Wafer-Scale Multilayer Fabrication of Silk Fibroin-Based Microelectronics
A KAIST research team developed a novel fabrication method for the multilayer processing of silk-based microelectronics. This technology for creating a biodegradable silk fibroin film allows microfabrication with polymer or metal structures manufactured from photolithography. It can be a key technology in the implementation of silk fibroin-based biodegradable electronic devices or localized drug delivery through silk fibroin patterns.
Silk fibroins are biocompatible, biodegradable, transparent, and flexible, which makes them excellent candidates for implantable biomedical devices, and they have also been used as biodegradable films and functional microstructures in biomedical applications. However, conventional microfabrication processes require strong etching solutions and solvents to modify the structure of silk fibroins.
To prevent the silk fibroin from being damaged during the process, Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team came up with a novel process, named aluminum hard mask on silk fibroin (AMoS), which is capable of micropatterning multiple layers composed of both fibroin and inorganic materials, such as metal and dielectrics with high-precision microscale alignment. The AMoS process can make silk fibroin patterns on devices, or make patterns on silk fibroin thin films with other materials by using photolithography, which is a core technology in the current microfabrication process.
The team successfully cultured primary neurons on the processed silk fibroin micro-patterns, and confirmed that silk fibroin has excellent biocompatibility before and after the fabrication process and that it also can be applied to implanted biological devices.
Through this technology, the team realized the multilayer micropatterning of fibroin films on a silk fibroin substrate and fabricated a biodegradable microelectric circuit consisting of resistors and silk fibroin dielectric capacitors in a silicon wafer with large areas.
They also used this technology to position the micro-pattern of the silk fibroin thin film closer to the flexible polymer-based brain electrode, and confirmed the dye molecules mounted on the silk fibroin were transferred successfully from the micropatterns.
Professor Lee said, “This technology facilitates wafer-scale, large-area processing of sensitive materials. We expect it to be applied to a wide range of biomedical devices in the future. Using the silk fibroin with micro-patterned brain electrodes can open up many new possibilities in research on brain circuits by mounting drugs that restrict or promote brain cell activities.”
This research, in collaboration with Dr. Nakwon Choi from KIST and led by PhD candidate Geon Kook, was published in ACS AMI (10.1021/acsami.8b13170) on January 16, 2019.
Figure 1. The cover page of ACS AMI
Figure 2. Fibroin microstructures and metal patterns on a fibroin produced by using the AMoS mask.
Figure 3. Biocompatibility assessment of the AMoS Process. Top: Schematics image of a) fibroin-coated silicon b) fibroin-pattered silicon and c) gold-patterned fibroin. Bottom: Representative confocal microscopy images of live (green) and dead (red) primary cortical neurons cultured on the substrates.
2019.03.15 View 23671 -
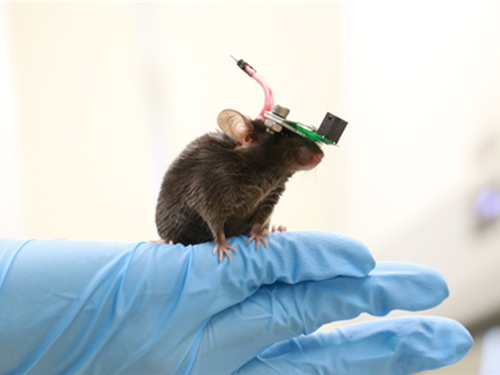 1g-Ultrasound System for the Brain Stimulation of a Freely-moving Mouse
A KAIST research team developed a light-weight capacitive micromachined ultrasonic transducer (CMUT) and succeeded in the ultrasound brain stimulation of a freely-moving mouse. With this lightweight and compact system, researchers can conduct a versatile set of in vivo experiments.
Conventional methods for stimulating a specific brain region, such as deep brain stimulation (DBS) and optogenetics technology, are highly invasive because they have to insert probes into a target brain, which makes them difficult to use for clinical application. While transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) are noninvasive, they have a wide range of stimulation and problems with in-depth stimulation, which makes them problematic for target-specific treatment.
Therefore, noninvasive and focused ultrasound stimulation technology is gaining a great deal of attention as a next-generation brain stimulation alternative. Since it is delivered noninvasively, it can be applied safely in humans as well as animal experiments. Focused ultrasound stimulation is more advantageous than conventional methods in terms of providing both local and deep stimulation.
Animal behavior experiments are essential for brain stimulation research; however, ultrasonic brain stimulation technology is currently in the early stages of development. So far, only research outcomes with fixed anesthetized mice have been studied because of the heavy ultrasonic device.
Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team reported a technology that can provide ultrasound stimulation to the brain of a freely-moving mouse through a microminiaturized ultrasound device.
The team studied miniaturization and ultra-lightweight CMUTs through microelectromechanical systems (MEMS) technology and designed a device suitable for behavior experiments. The device weighing less than 1g (around 0.05% of the mouse’s weight) has the center frequency, size, focal length, and ultrasonic intensity to fit a mouse’s dimensions.
To evaluate the performance of the ultrasonic device, the team stimulated the motor cortex of the mouse brain and observed the movement reaction of its forefoot. They also measured the electromyography (EMG) of the trapezius.
As a result, the team confirmed that their ultrasonic device can deliver ultrasound to a depth of 3-4mm in the mouse brain and stimulate an area of the mouse brain that represents 25% of its total size.
Based on this research, the team is investigating the effects of ultrasound on sleep by stimulating the brain of sleeping mice.
Professor Lee said, “Going beyond experimenting on fixed anesthetized mice, this research succeeded in the brain stimulation of a freely-moving mouse. We are planning to study mice with diseases, such as Parkinson’s disease, dementia, depression, and epilepsy. I believe that this basic research can contribute to treating human brain-related diseases through ultrasound brain stimulation.
This research, led by Masters candidates Hyunggug Kim and Seongyeon Kim, was published in Brain Stimulation (10.1016/j.brs.2018.11.007) on November 17, 2018.
Figure 1. The miniature transducer for the transcranial ultrasound of a freely-moving mouse
Figure 2. Its structure and simulated 2D beam profile in the axial ad radial directions
2019.03.13 View 10225
1g-Ultrasound System for the Brain Stimulation of a Freely-moving Mouse
A KAIST research team developed a light-weight capacitive micromachined ultrasonic transducer (CMUT) and succeeded in the ultrasound brain stimulation of a freely-moving mouse. With this lightweight and compact system, researchers can conduct a versatile set of in vivo experiments.
Conventional methods for stimulating a specific brain region, such as deep brain stimulation (DBS) and optogenetics technology, are highly invasive because they have to insert probes into a target brain, which makes them difficult to use for clinical application. While transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) are noninvasive, they have a wide range of stimulation and problems with in-depth stimulation, which makes them problematic for target-specific treatment.
Therefore, noninvasive and focused ultrasound stimulation technology is gaining a great deal of attention as a next-generation brain stimulation alternative. Since it is delivered noninvasively, it can be applied safely in humans as well as animal experiments. Focused ultrasound stimulation is more advantageous than conventional methods in terms of providing both local and deep stimulation.
Animal behavior experiments are essential for brain stimulation research; however, ultrasonic brain stimulation technology is currently in the early stages of development. So far, only research outcomes with fixed anesthetized mice have been studied because of the heavy ultrasonic device.
Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team reported a technology that can provide ultrasound stimulation to the brain of a freely-moving mouse through a microminiaturized ultrasound device.
The team studied miniaturization and ultra-lightweight CMUTs through microelectromechanical systems (MEMS) technology and designed a device suitable for behavior experiments. The device weighing less than 1g (around 0.05% of the mouse’s weight) has the center frequency, size, focal length, and ultrasonic intensity to fit a mouse’s dimensions.
To evaluate the performance of the ultrasonic device, the team stimulated the motor cortex of the mouse brain and observed the movement reaction of its forefoot. They also measured the electromyography (EMG) of the trapezius.
As a result, the team confirmed that their ultrasonic device can deliver ultrasound to a depth of 3-4mm in the mouse brain and stimulate an area of the mouse brain that represents 25% of its total size.
Based on this research, the team is investigating the effects of ultrasound on sleep by stimulating the brain of sleeping mice.
Professor Lee said, “Going beyond experimenting on fixed anesthetized mice, this research succeeded in the brain stimulation of a freely-moving mouse. We are planning to study mice with diseases, such as Parkinson’s disease, dementia, depression, and epilepsy. I believe that this basic research can contribute to treating human brain-related diseases through ultrasound brain stimulation.
This research, led by Masters candidates Hyunggug Kim and Seongyeon Kim, was published in Brain Stimulation (10.1016/j.brs.2018.11.007) on November 17, 2018.
Figure 1. The miniature transducer for the transcranial ultrasound of a freely-moving mouse
Figure 2. Its structure and simulated 2D beam profile in the axial ad radial directions
2019.03.13 View 10225