research
-
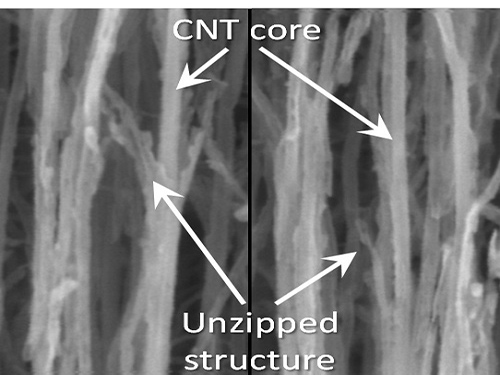 KAIST Team Develops Technology to Enable Unzipping of the Graphene Plane
Professor Sang-Wook Kim’s research team of the Material Science and Engineering Department has developed a technique, which enables unzipping of the graphene plane without uncontrollable damage. The research findings were published online on the January 22 issue of Nature Communications.
Graphene is a form of carbon in which its atoms form a honey-comb structure through chemical bonding. If this structure can be cut to a desired form, other carbon materials with nanostructure can be created. Many researchers have tried to obtain the accurate unzipping of graphene structures, but faced challenges doing so.
To break a very strong bond between carbon atoms, an equivalently strong chemical reaction must be induced. But the chemical reaction not only cuts out the desirable borders, but also damages the surrounding ones. Conventional techniques, which cut out graphene at once, damaged the chemical properties of the graphene structure after unzipping. This is similar to wearing out paper while manipulating it.
To solve this problem, the research team adopted “heteroatom doping.” The idea is similar to a sheet of paper being split following a groove drawn on the sheet. After making some regions of the structure unstable by doping other atoms such as nitrogen on a carbon plane, the regions are electrochemically stimulated to split the parts. Nitrogen or other atoms act as the groove on the grapheme plane.
The researchers finely controlled the amount of unzipping graphene by adjusting the amount of heteroatom dopants, from which they were able to create a quality nano graphene without any damage in its 2-dimensional crystalline structure. Using this technique, the researchers were able to obtain a capacitor with state-of-the-art energy transfer speed. The nano graphene can be combined with polymer, metal, and semiconductor nano molecules to form carbon composites.
Professor Kim said, “In order to commercialize this technique, heteroatom doping should be researched further. We plan to develop fabric-like carbon materials with excellent mechanical and electrical properties using this technique.”
Picture 1: Unzipped Carbon Nano Tube
Picture 2: Development of Nano Graphene from Carbon Nano Tube Using Heteroatom Dopants
Korean descriptions translated into English:
Unzipping Process of Graphene
Carbon Nano Tube → Nano Graphen
Heteroatom
This process is similar to a paper being split in two from a tiny hole punched therein.
2016.03.22 View 9624
KAIST Team Develops Technology to Enable Unzipping of the Graphene Plane
Professor Sang-Wook Kim’s research team of the Material Science and Engineering Department has developed a technique, which enables unzipping of the graphene plane without uncontrollable damage. The research findings were published online on the January 22 issue of Nature Communications.
Graphene is a form of carbon in which its atoms form a honey-comb structure through chemical bonding. If this structure can be cut to a desired form, other carbon materials with nanostructure can be created. Many researchers have tried to obtain the accurate unzipping of graphene structures, but faced challenges doing so.
To break a very strong bond between carbon atoms, an equivalently strong chemical reaction must be induced. But the chemical reaction not only cuts out the desirable borders, but also damages the surrounding ones. Conventional techniques, which cut out graphene at once, damaged the chemical properties of the graphene structure after unzipping. This is similar to wearing out paper while manipulating it.
To solve this problem, the research team adopted “heteroatom doping.” The idea is similar to a sheet of paper being split following a groove drawn on the sheet. After making some regions of the structure unstable by doping other atoms such as nitrogen on a carbon plane, the regions are electrochemically stimulated to split the parts. Nitrogen or other atoms act as the groove on the grapheme plane.
The researchers finely controlled the amount of unzipping graphene by adjusting the amount of heteroatom dopants, from which they were able to create a quality nano graphene without any damage in its 2-dimensional crystalline structure. Using this technique, the researchers were able to obtain a capacitor with state-of-the-art energy transfer speed. The nano graphene can be combined with polymer, metal, and semiconductor nano molecules to form carbon composites.
Professor Kim said, “In order to commercialize this technique, heteroatom doping should be researched further. We plan to develop fabric-like carbon materials with excellent mechanical and electrical properties using this technique.”
Picture 1: Unzipped Carbon Nano Tube
Picture 2: Development of Nano Graphene from Carbon Nano Tube Using Heteroatom Dopants
Korean descriptions translated into English:
Unzipping Process of Graphene
Carbon Nano Tube → Nano Graphen
Heteroatom
This process is similar to a paper being split in two from a tiny hole punched therein.
2016.03.22 View 9624 -
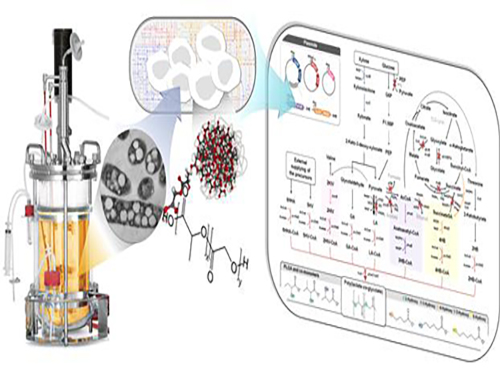 Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12760
Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12760 -
 K-Glass 3 Offers Users a Keyboard to Type Text
KAIST researchers upgraded their smart glasses with a low-power multicore processor to employ stereo vision and deep-learning algorithms, making the user interface and experience more intuitive and convenient.
K-Glass, smart glasses reinforced with augmented reality (AR) that were first developed by KAIST in 2014, with the second version released in 2015, is back with an even stronger model. The latest version, which KAIST researchers are calling K-Glass 3, allows users to text a message or type in key words for Internet surfing by offering a virtual keyboard for text and even one for a piano.
Currently, most wearable head-mounted displays (HMDs) suffer from a lack of rich user interfaces, short battery lives, and heavy weight. Some HMDs, such as Google Glass, use a touch panel and voice commands as an interface, but they are considered merely an extension of smartphones and are not optimized for wearable smart glasses. Recently, gaze recognition was proposed for HMDs including K-Glass 2, but gaze cannot be realized as a natural user interface (UI) and experience (UX) due to its limited interactivity and lengthy gaze-calibration time, which can be up to several minutes.
As a solution, Professor Hoi-Jun Yoo and his team from the Electrical Engineering Department recently developed K-Glass 3 with a low-power natural UI and UX processor. This processor is composed of a pre-processing core to implement stereo vision, seven deep-learning cores to accelerate real-time scene recognition within 33 milliseconds, and one rendering engine for the display.
The stereo-vision camera, located on the front of K-Glass 3, works in a manner similar to three dimension (3D) sensing in human vision. The camera’s two lenses, displayed horizontally from one another just like depth perception produced by left and right eyes, take pictures of the same objects or scenes and combine these two different images to extract spatial depth information, which is necessary to reconstruct 3D environments. The camera’s vision algorithm has an energy efficiency of 20 milliwatts on average, allowing it to operate in the Glass more than 24 hours without interruption.
The research team adopted deep-learning-multi core technology dedicated for mobile devices. This technology has greatly improved the Glass’s recognition accuracy with images and speech, while shortening the time needed to process and analyze data. In addition, the Glass’s multi-core processor is advanced enough to become idle when it detects no motion from users. Instead, it executes complex deep-learning algorithms with a minimal power to achieve high performance.
Professor Yoo said, “We have succeeded in fabricating a low-power multi-core processer that consumes only 126 milliwatts of power with a high efficiency rate. It is essential to develop a smaller, lighter, and low-power processor if we want to incorporate the widespread use of smart glasses and wearable devices into everyday life. K-Glass 3’s more intuitive UI and convenient UX permit users to enjoy enhanced AR experiences such as a keyboard or a better, more responsive mouse.”
Along with the research team, UX Factory, a Korean UI and UX developer, participated in the K-Glass 3 project.
These research results entitled “A 126.1mW Real-Time Natural UI/UX Processor with Embedded Deep-Learning Core for Low-Power Smart Glasses” (lead author: Seong-Wook Park, a doctoral student in the Electrical Engineering Department, KAIST) were presented at the 2016 IEEE (Institute of Electrical and Electronics Engineers) International Solid-State Circuits Conference (ISSCC) that took place January 31-February 4, 2016 in San Francisco, California.
YouTube Link: https://youtu.be/If_anx5NerQ
Figure 1: K-Glass 3
K-Glass 3 is equipped with a stereo camera, dual microphones, a WiFi module, and eight batteries to offer higher recognition accuracy and enhanced augmented reality experiences than previous models.
Figure 2: Architecture of the Low-Power Multi-Core Processor
K-Glass 3’s processor is designed to include several cores for pre-processing, deep-learning, and graphic rendering.
Figure 3: Virtual Text and Piano Keyboard
K-Glass 3 can detect hands and recognize their movements to provide users with such augmented reality applications as a virtual text or piano keyboard.
2016.02.26 View 14156
K-Glass 3 Offers Users a Keyboard to Type Text
KAIST researchers upgraded their smart glasses with a low-power multicore processor to employ stereo vision and deep-learning algorithms, making the user interface and experience more intuitive and convenient.
K-Glass, smart glasses reinforced with augmented reality (AR) that were first developed by KAIST in 2014, with the second version released in 2015, is back with an even stronger model. The latest version, which KAIST researchers are calling K-Glass 3, allows users to text a message or type in key words for Internet surfing by offering a virtual keyboard for text and even one for a piano.
Currently, most wearable head-mounted displays (HMDs) suffer from a lack of rich user interfaces, short battery lives, and heavy weight. Some HMDs, such as Google Glass, use a touch panel and voice commands as an interface, but they are considered merely an extension of smartphones and are not optimized for wearable smart glasses. Recently, gaze recognition was proposed for HMDs including K-Glass 2, but gaze cannot be realized as a natural user interface (UI) and experience (UX) due to its limited interactivity and lengthy gaze-calibration time, which can be up to several minutes.
As a solution, Professor Hoi-Jun Yoo and his team from the Electrical Engineering Department recently developed K-Glass 3 with a low-power natural UI and UX processor. This processor is composed of a pre-processing core to implement stereo vision, seven deep-learning cores to accelerate real-time scene recognition within 33 milliseconds, and one rendering engine for the display.
The stereo-vision camera, located on the front of K-Glass 3, works in a manner similar to three dimension (3D) sensing in human vision. The camera’s two lenses, displayed horizontally from one another just like depth perception produced by left and right eyes, take pictures of the same objects or scenes and combine these two different images to extract spatial depth information, which is necessary to reconstruct 3D environments. The camera’s vision algorithm has an energy efficiency of 20 milliwatts on average, allowing it to operate in the Glass more than 24 hours without interruption.
The research team adopted deep-learning-multi core technology dedicated for mobile devices. This technology has greatly improved the Glass’s recognition accuracy with images and speech, while shortening the time needed to process and analyze data. In addition, the Glass’s multi-core processor is advanced enough to become idle when it detects no motion from users. Instead, it executes complex deep-learning algorithms with a minimal power to achieve high performance.
Professor Yoo said, “We have succeeded in fabricating a low-power multi-core processer that consumes only 126 milliwatts of power with a high efficiency rate. It is essential to develop a smaller, lighter, and low-power processor if we want to incorporate the widespread use of smart glasses and wearable devices into everyday life. K-Glass 3’s more intuitive UI and convenient UX permit users to enjoy enhanced AR experiences such as a keyboard or a better, more responsive mouse.”
Along with the research team, UX Factory, a Korean UI and UX developer, participated in the K-Glass 3 project.
These research results entitled “A 126.1mW Real-Time Natural UI/UX Processor with Embedded Deep-Learning Core for Low-Power Smart Glasses” (lead author: Seong-Wook Park, a doctoral student in the Electrical Engineering Department, KAIST) were presented at the 2016 IEEE (Institute of Electrical and Electronics Engineers) International Solid-State Circuits Conference (ISSCC) that took place January 31-February 4, 2016 in San Francisco, California.
YouTube Link: https://youtu.be/If_anx5NerQ
Figure 1: K-Glass 3
K-Glass 3 is equipped with a stereo camera, dual microphones, a WiFi module, and eight batteries to offer higher recognition accuracy and enhanced augmented reality experiences than previous models.
Figure 2: Architecture of the Low-Power Multi-Core Processor
K-Glass 3’s processor is designed to include several cores for pre-processing, deep-learning, and graphic rendering.
Figure 3: Virtual Text and Piano Keyboard
K-Glass 3 can detect hands and recognize their movements to provide users with such augmented reality applications as a virtual text or piano keyboard.
2016.02.26 View 14156 -
 A Firefighter Drone That Flies and Crawls Up Walls
KAIST researchers developed a wall-climbing scout drone to fight fires in high-rises, finding the source of the fires and locating people trapped inside.
The 1974 American disaster film Towering Inferno depicted well the earnest struggles of firefighters engaged in ending a fire at a 138-story skyscraper. To this day, fires at high-rise buildings are considered one of the most dangerous disasters.
Skyscraper fires are particularly difficult to contain because of their ability to spread rapidly in high-occupant density spaces and the challenge of fighting fires in the buildings’ complex vertical structure. Accessibility to skyscrapers at the time of the fire is limited, and it is hard to assess the initial situation.
A research team at KAIST led by Professor Hyun Myung of the Civil and Environmental Engineering Department developed an unmanned aerial vehicle, named the Fireproof Aerial RObot System (FAROS), which detects fires in skyscrapers, searches the inside of the building, and transfers data in real time from fire scenes to the ground station.
As an extended version of Climbing Aerial RObot System (CAROS) that was created in 2014 by the research team, the FAROS can also fly and climb walls.
The FAROS, whose movements rely on a quadrotor system, can freely change its flight mode into a spider’s crawling on walls, and vice versa, facilitating unimpeded navigation in the labyrinth of narrow spaces filled with debris and rubble inside the blazing building.
The drone “estimates” its pose by utilizing a 2-D laser scanner, an altimeter, and an Inertia Measurement Unit sensor to navigate autonomously. With the localization result and using a thermal-imaging camera to recognize objects or people inside a building, the FAROS can also detect and find the fire-ignition point by employing dedicated image-processing technology.
The FAROS is fireproof and flame-retardant. The drone’s body is covered with aramid fibers to protect its electric and mechanical components from the direct effects of the flame. The aramid fiber skin also has a buffer of air underneath it, and a thermoelectric cooling system based on the Peltier effect to help maintain the air layer within a specific temperature range.
The research team demonstrated the feasibility of the localization system and wall-climbing mechanism in a smoky indoor environment. The fireproof test showed that the drone could endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
Professor Myung said, “As cities become more crowded with skyscrapers and super structures, fire incidents in these high-rise buildings are life-threatening massive disasters. The FAROS can be aptly deployed to the disaster site at an early stage of such incidents to minimize the damage and maximize the safety and efficiency of rescue mission.”
The research team has recently started to enhance the performance of the fireproof design for the exteroceptive sensors including a 2-D laser scanner and a thermal-imaging camera because those sensors could be more exposed to fire than other inside sensors and electric components.
This research was funded by the KAIST Initiative for Disaster Studies and the KAIST Institute.
YouTube link: https://youtu.be/gPNRZi0EPQw
Picture 1: Demonstration of Wall-climbing
The Fireproof Aerial RObot System (FAROS) is a wall-climbing scout drone developed to conduct explorations into the site of skyscraper fires. It has an ability to climb walls in smoky, narrow spaces inside buildings.
Figure 2: An Ability to Withstand Fires
The FAROS can endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
2016.01.20 View 16213
A Firefighter Drone That Flies and Crawls Up Walls
KAIST researchers developed a wall-climbing scout drone to fight fires in high-rises, finding the source of the fires and locating people trapped inside.
The 1974 American disaster film Towering Inferno depicted well the earnest struggles of firefighters engaged in ending a fire at a 138-story skyscraper. To this day, fires at high-rise buildings are considered one of the most dangerous disasters.
Skyscraper fires are particularly difficult to contain because of their ability to spread rapidly in high-occupant density spaces and the challenge of fighting fires in the buildings’ complex vertical structure. Accessibility to skyscrapers at the time of the fire is limited, and it is hard to assess the initial situation.
A research team at KAIST led by Professor Hyun Myung of the Civil and Environmental Engineering Department developed an unmanned aerial vehicle, named the Fireproof Aerial RObot System (FAROS), which detects fires in skyscrapers, searches the inside of the building, and transfers data in real time from fire scenes to the ground station.
As an extended version of Climbing Aerial RObot System (CAROS) that was created in 2014 by the research team, the FAROS can also fly and climb walls.
The FAROS, whose movements rely on a quadrotor system, can freely change its flight mode into a spider’s crawling on walls, and vice versa, facilitating unimpeded navigation in the labyrinth of narrow spaces filled with debris and rubble inside the blazing building.
The drone “estimates” its pose by utilizing a 2-D laser scanner, an altimeter, and an Inertia Measurement Unit sensor to navigate autonomously. With the localization result and using a thermal-imaging camera to recognize objects or people inside a building, the FAROS can also detect and find the fire-ignition point by employing dedicated image-processing technology.
The FAROS is fireproof and flame-retardant. The drone’s body is covered with aramid fibers to protect its electric and mechanical components from the direct effects of the flame. The aramid fiber skin also has a buffer of air underneath it, and a thermoelectric cooling system based on the Peltier effect to help maintain the air layer within a specific temperature range.
The research team demonstrated the feasibility of the localization system and wall-climbing mechanism in a smoky indoor environment. The fireproof test showed that the drone could endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
Professor Myung said, “As cities become more crowded with skyscrapers and super structures, fire incidents in these high-rise buildings are life-threatening massive disasters. The FAROS can be aptly deployed to the disaster site at an early stage of such incidents to minimize the damage and maximize the safety and efficiency of rescue mission.”
The research team has recently started to enhance the performance of the fireproof design for the exteroceptive sensors including a 2-D laser scanner and a thermal-imaging camera because those sensors could be more exposed to fire than other inside sensors and electric components.
This research was funded by the KAIST Initiative for Disaster Studies and the KAIST Institute.
YouTube link: https://youtu.be/gPNRZi0EPQw
Picture 1: Demonstration of Wall-climbing
The Fireproof Aerial RObot System (FAROS) is a wall-climbing scout drone developed to conduct explorations into the site of skyscraper fires. It has an ability to climb walls in smoky, narrow spaces inside buildings.
Figure 2: An Ability to Withstand Fires
The FAROS can endure the heat of over 1,000° Celsius from butane gas and ethanol aerosol flames for over one minute.
2016.01.20 View 16213 -
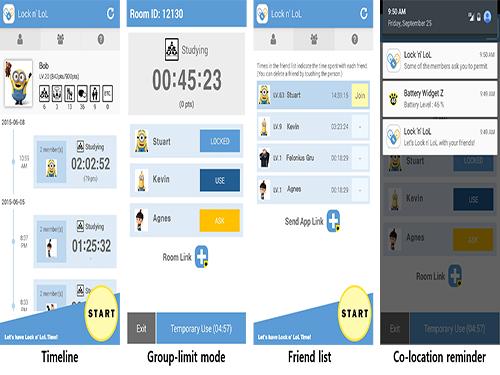 An App to Digitally Detox from Smartphone Addiction: Lock n' LOL
KAIST researchers have developed an application that helps people restrain themselves from using smartphones during meetings or social gatherings.
The app’s group limit mode enforces users to curtail their smartphone usage through peer-pressure while offering flexibility to use the phone in an emergency.
When a fake phone company released its line of products, NoPhones, a thin, rectangular-shaped plastic block that looked just like a smartphone but did not function, many doubted that the simulated smartphones would find any users. Surprisingly, close to 4,000 fake phones were sold to consumers who wanted to curb their phone usage.
As smartphones penetrate every facet of our daily lives, a growing number of people have expressed concern about distractions or even the addictions they suffer from overusing smartphones.
Professor Uichin Lee of the Department of Knowledge Service Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have recently introduced a solution to this problem by developing an application, Lock n’ LoL (Lock Your Smartphone and Laugh Out Loud), to help people lock their smartphones altogether and keep them from using the phone while engaged in social activities such as meetings, conferences, and discussions.
Researchers note that the overuse of smartphones often results from users’ habitual checking of messages, emails, or other online contents such as status updates in social networking service (SNS). External stimuli, for example, notification alarms, add to smartphone distractions and interruptions in group interactions.
The Lock n’ LoL allows users to create a new room or join an existing room. The users then invite meeting participants or friends to the room and share its ID with them to enact the Group Limit (lock) mode. When phones are in the lock mode, all alarms and notifications are automatically muted, and users must ask permission to unlock their phones. However, in an emergency, users can access their phones for accumulative five minutes in a temporary unlimit mode.
In addition, the app’s Co-location Reminder detects and lists nearby users to encourage app users to limit their phone use. The Lock n’ LoL also displays important statistics to monitor users’ behavior such as the current week’s total limit time, the weekly average usage time, top friends ranked by time spent together, and top activities in which the users participated.
Professor Lee said,
“We conducted the Lock n’ LoL campaign throughout the campus for one month this year with 1,000 students participating. As a result, we discovered that students accumulated more than 10,000 free hours from using the app on their smartphones. The students said that they were able to focus more on their group activities. In an age of the Internet of Things, we expect that the adverse effects of mobile distractions and addictions will emerge as a social concern, and our Lock n’ LoL is a key effort to address this issue.”
He added, “This app will certainly help family members to interact more with each other during the holiday season.”
The Lock n’ LoL is available for free download on the App Store and Google Play: https://itunes.apple.com/lc/app/lock-n-lol/id1030287673?mt=8.
YouTube link: https://youtu.be/1wY2pI9qFYM
Figure 1: User Interfaces of Lock n’ LoL
This shows the final design of Lock n’ LoL, which consists of three tabs: My Info, Friends, and Group Limit Mode. Users can activate the limit mode by clicking the start button at the bottom of the screen.
Figure 2: Statistics of Field Deployment
This shows the deployment summary of Lock n’ LoL campaign in May 2015.
2015.12.17 View 10871
An App to Digitally Detox from Smartphone Addiction: Lock n' LOL
KAIST researchers have developed an application that helps people restrain themselves from using smartphones during meetings or social gatherings.
The app’s group limit mode enforces users to curtail their smartphone usage through peer-pressure while offering flexibility to use the phone in an emergency.
When a fake phone company released its line of products, NoPhones, a thin, rectangular-shaped plastic block that looked just like a smartphone but did not function, many doubted that the simulated smartphones would find any users. Surprisingly, close to 4,000 fake phones were sold to consumers who wanted to curb their phone usage.
As smartphones penetrate every facet of our daily lives, a growing number of people have expressed concern about distractions or even the addictions they suffer from overusing smartphones.
Professor Uichin Lee of the Department of Knowledge Service Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have recently introduced a solution to this problem by developing an application, Lock n’ LoL (Lock Your Smartphone and Laugh Out Loud), to help people lock their smartphones altogether and keep them from using the phone while engaged in social activities such as meetings, conferences, and discussions.
Researchers note that the overuse of smartphones often results from users’ habitual checking of messages, emails, or other online contents such as status updates in social networking service (SNS). External stimuli, for example, notification alarms, add to smartphone distractions and interruptions in group interactions.
The Lock n’ LoL allows users to create a new room or join an existing room. The users then invite meeting participants or friends to the room and share its ID with them to enact the Group Limit (lock) mode. When phones are in the lock mode, all alarms and notifications are automatically muted, and users must ask permission to unlock their phones. However, in an emergency, users can access their phones for accumulative five minutes in a temporary unlimit mode.
In addition, the app’s Co-location Reminder detects and lists nearby users to encourage app users to limit their phone use. The Lock n’ LoL also displays important statistics to monitor users’ behavior such as the current week’s total limit time, the weekly average usage time, top friends ranked by time spent together, and top activities in which the users participated.
Professor Lee said,
“We conducted the Lock n’ LoL campaign throughout the campus for one month this year with 1,000 students participating. As a result, we discovered that students accumulated more than 10,000 free hours from using the app on their smartphones. The students said that they were able to focus more on their group activities. In an age of the Internet of Things, we expect that the adverse effects of mobile distractions and addictions will emerge as a social concern, and our Lock n’ LoL is a key effort to address this issue.”
He added, “This app will certainly help family members to interact more with each other during the holiday season.”
The Lock n’ LoL is available for free download on the App Store and Google Play: https://itunes.apple.com/lc/app/lock-n-lol/id1030287673?mt=8.
YouTube link: https://youtu.be/1wY2pI9qFYM
Figure 1: User Interfaces of Lock n’ LoL
This shows the final design of Lock n’ LoL, which consists of three tabs: My Info, Friends, and Group Limit Mode. Users can activate the limit mode by clicking the start button at the bottom of the screen.
Figure 2: Statistics of Field Deployment
This shows the deployment summary of Lock n’ LoL campaign in May 2015.
2015.12.17 View 10871 -
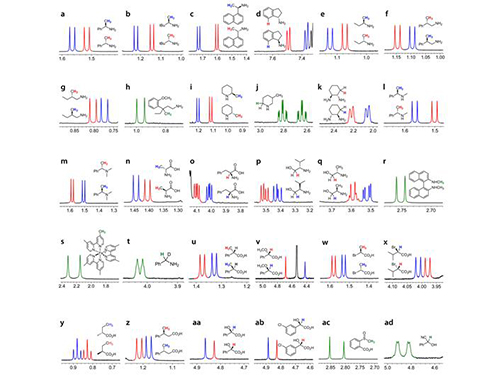 KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 12549
KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 12549 -
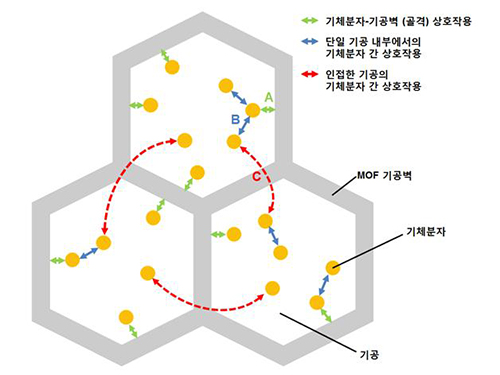 A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 8930
A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 8930 -
 Using Light to Treat Alzheimer's Disease
Medical application of photoactive chemicals offers a promising therapeutic strategy for neurodegenerative diseases.
A research team jointly led by Professor Chan Beum Park of the Materials Science and Engineering Department at KAIST and Dr. Kwon Yu from the Bionano Center at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) conducted research to suppress an abnormal assembly of beta-amyloids, a protein commonly found in the brain, by using photo-excited porphyrins.
Beta-amyloid plaques are known to cause Alzheimer's disease. This research finding suggests new ways to treat neurodegenerative illnesses including Alzheimer's disease. It was published online as the lead article in the September 21th issue of Angewandte Chemie. The title of the article is “Photo-excited Porphyrins as a Strong Suppressor of ß-Amyloid Aggregation and Synaptic Toxicity.”
Light-induced treatments using organic photosensitizers have advantages to managing the treatment in time and area. In the case of cancer treatments, doctors use photodynamic therapies where a patient is injected with an organic photosensitizer, and a light is shed on the patient’s lesion. However, such therapies had never been employed to treat neurodegenerative diseases.
Alzheimer's starts when a protein called beta-amyloid is created and deposited in a patient’s brain. The abnormally folded protein created this way harms the brain cells by inducing the degradation of brain functions, for example, dementia. If beta-amyloid creation can be suppressed at an early stage, the formation of amyloid deposits will stop. This could prevent Alzheimer’s disease or halt its progress.
The research team effectively prevented the buildup of beta-amyloids by using blue LED lights and a porphyrin inducer, which is a biocompatible organic compound. By absorbing light energy, a photosensitizer such as porphyrin reaches the excitation state. Active oxygen is created as the porphyrin returns to its ground state. The active oxygen oxidizes a beta-amyloid monomer, and by combining with it, disturbs its assembly.
The technique was tested on drosophilae or fruit flies, which were produced to model Alzheimer on invertebrates. The research showed that symptoms of Alzheimer's disease in the fruit flies such as damage on synapse and muscle, neuronal apoptosis, degradation in motility, and decreased longevity were alleviated. Treatments with light provide additional benefits: less medication is needed than other drug treatments, and there are fewer side effects. When developed, photodynamic therapy will be used widely for this reason.
Professor Park said, “This work has significance as it was the first case to use light and photosensitizers to stop deposits of beta-amyloids. We plan to carry the research further by testing compatibility with other organic and inorganic photosensitizers and by changing the subject of photodynamic therapy to vertebrate such as mice.”
Picture 1 – Deposits of Beta-Amyloid in Fruit Flies Stopped by Using Porphyrin and Blue LED Lights
Picture 2 – The Research Finding Published as the Lead Article in Angewandte Chemie (September 2015)
2015.11.11 View 12040
Using Light to Treat Alzheimer's Disease
Medical application of photoactive chemicals offers a promising therapeutic strategy for neurodegenerative diseases.
A research team jointly led by Professor Chan Beum Park of the Materials Science and Engineering Department at KAIST and Dr. Kwon Yu from the Bionano Center at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) conducted research to suppress an abnormal assembly of beta-amyloids, a protein commonly found in the brain, by using photo-excited porphyrins.
Beta-amyloid plaques are known to cause Alzheimer's disease. This research finding suggests new ways to treat neurodegenerative illnesses including Alzheimer's disease. It was published online as the lead article in the September 21th issue of Angewandte Chemie. The title of the article is “Photo-excited Porphyrins as a Strong Suppressor of ß-Amyloid Aggregation and Synaptic Toxicity.”
Light-induced treatments using organic photosensitizers have advantages to managing the treatment in time and area. In the case of cancer treatments, doctors use photodynamic therapies where a patient is injected with an organic photosensitizer, and a light is shed on the patient’s lesion. However, such therapies had never been employed to treat neurodegenerative diseases.
Alzheimer's starts when a protein called beta-amyloid is created and deposited in a patient’s brain. The abnormally folded protein created this way harms the brain cells by inducing the degradation of brain functions, for example, dementia. If beta-amyloid creation can be suppressed at an early stage, the formation of amyloid deposits will stop. This could prevent Alzheimer’s disease or halt its progress.
The research team effectively prevented the buildup of beta-amyloids by using blue LED lights and a porphyrin inducer, which is a biocompatible organic compound. By absorbing light energy, a photosensitizer such as porphyrin reaches the excitation state. Active oxygen is created as the porphyrin returns to its ground state. The active oxygen oxidizes a beta-amyloid monomer, and by combining with it, disturbs its assembly.
The technique was tested on drosophilae or fruit flies, which were produced to model Alzheimer on invertebrates. The research showed that symptoms of Alzheimer's disease in the fruit flies such as damage on synapse and muscle, neuronal apoptosis, degradation in motility, and decreased longevity were alleviated. Treatments with light provide additional benefits: less medication is needed than other drug treatments, and there are fewer side effects. When developed, photodynamic therapy will be used widely for this reason.
Professor Park said, “This work has significance as it was the first case to use light and photosensitizers to stop deposits of beta-amyloids. We plan to carry the research further by testing compatibility with other organic and inorganic photosensitizers and by changing the subject of photodynamic therapy to vertebrate such as mice.”
Picture 1 – Deposits of Beta-Amyloid in Fruit Flies Stopped by Using Porphyrin and Blue LED Lights
Picture 2 – The Research Finding Published as the Lead Article in Angewandte Chemie (September 2015)
2015.11.11 View 12040 -
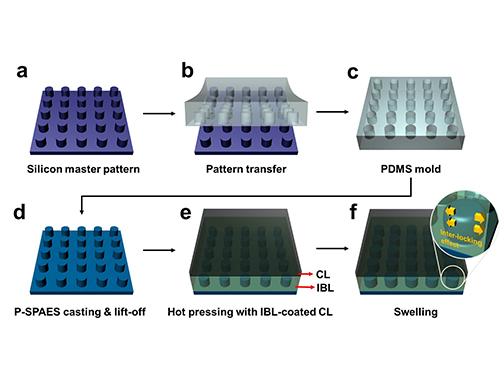 Membrane
Scientists at KAIST have developed a new way of making fuel cell membranes using nanoscale fasteners, paving the way for lower-cost, higher-efficiency and more easily manufactured fuel cells.
The internal workings of fuel cells vary, but basically all types mix hydrogen and oxygen to produce a chemical reaction that delivers usable electricity and exhausts ordinary water as a by-product. One of the most efficient types is the proton exchange membrane (PEM) fuel cell, which operates at low enough temperatures to be used in homes and vehicles.
To generate electricity, PEM fuel cells rely on two chemical compartments separated by a permeable catalyst membrane. This membrane acts as an electrolyte; a negative electrode is bonded to one side of the membrane and a positive electrode is bonded to the other. The electrolyte membrane is often based on a polymer of perfluorosulfonic acid. Due to its high cost, however, a less expensive hydrocarbon-based electrolyte membrane has attracted interest in this technology sector.
Until now, the challenge in adopting such a hydrocarbon membrane has been that the interface between the electrode and hydrocarbon membrane is weak. This causes the membrane to delaminate relatively easily, falling apart and losing efficiency with use.
Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have developed a new fastening system that bonds the two materials mechanically rather than chemically. This opens the way to the development of fuel cell membranes that are less expensive, easier to manufacture, stronger and more efficient.
The researchers achieved this by moulding a pattern of tiny cylindrical pillars on the face of the hydrocarbon membrane. The pillars protrude into a softened skin of the electrode with heat. The mechanical bond sets and strengthens as the material cools and absorbs water. The pillar-patterned hydrocarbon membrane is cast using silicone moulds.
Professor Kim said, “This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers.”
The new interlocking method also appears to offer a way to bond many types of hydrocarbon membranes that, until now, have been rejected because they couldn’t be fastened robustly. This would make hydrocarbon membranes practical for a number of applications beyond fuel cells such as rechargeable “redox flow” batteries.
The research team is now developing a stronger and more scalable interlocking interface for their nanoscale fasteners.
Picture: Schematic Diagram of the Fabrication of the Pillar P-SPAES Membrane and Its Working Principle of Interlocking Effects
2015.11.06 View 10626
Membrane
Scientists at KAIST have developed a new way of making fuel cell membranes using nanoscale fasteners, paving the way for lower-cost, higher-efficiency and more easily manufactured fuel cells.
The internal workings of fuel cells vary, but basically all types mix hydrogen and oxygen to produce a chemical reaction that delivers usable electricity and exhausts ordinary water as a by-product. One of the most efficient types is the proton exchange membrane (PEM) fuel cell, which operates at low enough temperatures to be used in homes and vehicles.
To generate electricity, PEM fuel cells rely on two chemical compartments separated by a permeable catalyst membrane. This membrane acts as an electrolyte; a negative electrode is bonded to one side of the membrane and a positive electrode is bonded to the other. The electrolyte membrane is often based on a polymer of perfluorosulfonic acid. Due to its high cost, however, a less expensive hydrocarbon-based electrolyte membrane has attracted interest in this technology sector.
Until now, the challenge in adopting such a hydrocarbon membrane has been that the interface between the electrode and hydrocarbon membrane is weak. This causes the membrane to delaminate relatively easily, falling apart and losing efficiency with use.
Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have developed a new fastening system that bonds the two materials mechanically rather than chemically. This opens the way to the development of fuel cell membranes that are less expensive, easier to manufacture, stronger and more efficient.
The researchers achieved this by moulding a pattern of tiny cylindrical pillars on the face of the hydrocarbon membrane. The pillars protrude into a softened skin of the electrode with heat. The mechanical bond sets and strengthens as the material cools and absorbs water. The pillar-patterned hydrocarbon membrane is cast using silicone moulds.
Professor Kim said, “This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers.”
The new interlocking method also appears to offer a way to bond many types of hydrocarbon membranes that, until now, have been rejected because they couldn’t be fastened robustly. This would make hydrocarbon membranes practical for a number of applications beyond fuel cells such as rechargeable “redox flow” batteries.
The research team is now developing a stronger and more scalable interlocking interface for their nanoscale fasteners.
Picture: Schematic Diagram of the Fabrication of the Pillar P-SPAES Membrane and Its Working Principle of Interlocking Effects
2015.11.06 View 10626 -
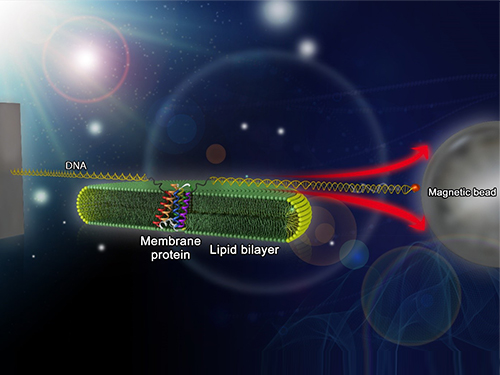 Mapping the Folding Process of a Single Membrane Protein
KAIST and UCLA scientists were able to observe an individual membrane protein fold and unfold by pulling and releasing magnetically trapped protein molecules.
Proteins are huge molecules containing hundreds to thousands of atoms that adopt a unique three dimensional structure, placing chemical groups in just the right place to catalyze reactions or build cellular structures. How all those atoms manage to find the right location - the so-called folding problem - has fascinated molecular biologists since the first structures were seen in the 1950s. Moreover, folding has important medical implications because most genetic defects cause protein misfolding.
About a third of all proteins float around in the cell membrane where they ensure the right chemicals get in the cell in the right amounts. Membrane proteins also provide key information links between the cell and its environment. Indeed, most drugs target membrane proteins. Nevertheless, the folding of membrane proteins has been particularly difficult to study and has rarely been studied in natural environments, leaving the folding process for a large fraction of the protein universe still largely cloaked in mystery.
In a recent issue of Nature Chemical Biology, published on October 19, 2015, a research team led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and James U. Bowie of the Department of Chemistry and Biochemistry at the University of California, Los Angeles (UCLA), report a new method for manipulating the folding of membrane proteins in a membrane environment using a tool called a magnetic tweezer.
Researchers first attach long DNA handles to the ends of the protein. One handle is attached to a glass surface and the other to a magnetic bead. Using a magnet, they can essentially grab the protein and pull on it, inducing it to unfold. By playing with the bead attached to the protein, they can force the protein to unfold or allow it to refold, and watch all this happening by 3D-tracking of the magnetic bead. With this novel strategy, they were able to quantitatively map the folding energy landscape, the folding kinetic rate, and folding intermediates of a membrane protein in a membrane environment for the first time.
“I have been dreaming about this experiment for a decade. To see it work so well is really gratifying,” said Dr. Bowie.
One of the major surprises in the study was that essentially all the atoms of the protein jump into the correct structure together. The researchers expected that the protein structure would come together in a more piecemeal fashion, with different parts of the structure forming separately, but that was not the case. It is possible that nature evolved such a smooth, highly cooperative folding process to prevent partially folded forms that could get into trouble in the crowded cell membrane. On the other hand, the cooperative folding seen here might not apply to other membrane proteins.
“We need to look at more proteins. The technique developed here may allow us to do just that,” said Dr. Yoon.
The single molecule mechanical manipulation technique could enable detailed folding studies of many other membrane proteins. A major barrier to the study of membrane proteins previously is that the proteins tend to stick together and get tangled up, as computer cords lying at your feet tend to do. With the tweezer technique used in this work, the protein cords are held apart from other cords so they can’t get knotted up. It is hoped that the new approach will open up an important part of the protein universe to scrutiny, including many proteins that become misfolded in disease states.
The title of the research paper is “Mapping the energy landscape for second-stage folding of a single membrane protein” (DOI: 10.1038/nchembio.1939).
Picture: Single-molecule magnetic tweezers that induce mechanical unfolding and refolding of a single membrane protein. Since the force applied is parallel to the biological lipid membrane, the unfolding and refolding processes occur within the membrane.
2015.10.20 View 10554
Mapping the Folding Process of a Single Membrane Protein
KAIST and UCLA scientists were able to observe an individual membrane protein fold and unfold by pulling and releasing magnetically trapped protein molecules.
Proteins are huge molecules containing hundreds to thousands of atoms that adopt a unique three dimensional structure, placing chemical groups in just the right place to catalyze reactions or build cellular structures. How all those atoms manage to find the right location - the so-called folding problem - has fascinated molecular biologists since the first structures were seen in the 1950s. Moreover, folding has important medical implications because most genetic defects cause protein misfolding.
About a third of all proteins float around in the cell membrane where they ensure the right chemicals get in the cell in the right amounts. Membrane proteins also provide key information links between the cell and its environment. Indeed, most drugs target membrane proteins. Nevertheless, the folding of membrane proteins has been particularly difficult to study and has rarely been studied in natural environments, leaving the folding process for a large fraction of the protein universe still largely cloaked in mystery.
In a recent issue of Nature Chemical Biology, published on October 19, 2015, a research team led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and James U. Bowie of the Department of Chemistry and Biochemistry at the University of California, Los Angeles (UCLA), report a new method for manipulating the folding of membrane proteins in a membrane environment using a tool called a magnetic tweezer.
Researchers first attach long DNA handles to the ends of the protein. One handle is attached to a glass surface and the other to a magnetic bead. Using a magnet, they can essentially grab the protein and pull on it, inducing it to unfold. By playing with the bead attached to the protein, they can force the protein to unfold or allow it to refold, and watch all this happening by 3D-tracking of the magnetic bead. With this novel strategy, they were able to quantitatively map the folding energy landscape, the folding kinetic rate, and folding intermediates of a membrane protein in a membrane environment for the first time.
“I have been dreaming about this experiment for a decade. To see it work so well is really gratifying,” said Dr. Bowie.
One of the major surprises in the study was that essentially all the atoms of the protein jump into the correct structure together. The researchers expected that the protein structure would come together in a more piecemeal fashion, with different parts of the structure forming separately, but that was not the case. It is possible that nature evolved such a smooth, highly cooperative folding process to prevent partially folded forms that could get into trouble in the crowded cell membrane. On the other hand, the cooperative folding seen here might not apply to other membrane proteins.
“We need to look at more proteins. The technique developed here may allow us to do just that,” said Dr. Yoon.
The single molecule mechanical manipulation technique could enable detailed folding studies of many other membrane proteins. A major barrier to the study of membrane proteins previously is that the proteins tend to stick together and get tangled up, as computer cords lying at your feet tend to do. With the tweezer technique used in this work, the protein cords are held apart from other cords so they can’t get knotted up. It is hoped that the new approach will open up an important part of the protein universe to scrutiny, including many proteins that become misfolded in disease states.
The title of the research paper is “Mapping the energy landscape for second-stage folding of a single membrane protein” (DOI: 10.1038/nchembio.1939).
Picture: Single-molecule magnetic tweezers that induce mechanical unfolding and refolding of a single membrane protein. Since the force applied is parallel to the biological lipid membrane, the unfolding and refolding processes occur within the membrane.
2015.10.20 View 10554 -
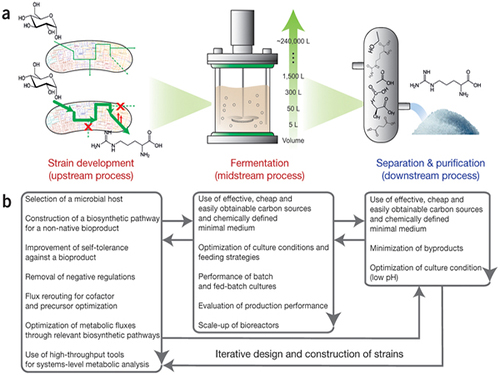 Establishment of System Metabolic Engineering Strategies
Although conventional petrochemical processes have generated chemicals and materials which have been useful to mankind, they have also triggered a variety of environmental problems including climate change and relied too much on nonrenewable natural resources. To ameliorate this, researchers have actively pursued the development of industrial microbial strains around the globe in order to overproduce industrially useful chemicals and materials from microbes using renewable biomass. This discipline is called metabolic engineering.
Thanks to advances in genetic engineering and our knowledge of cellular metabolism, conventional metabolic engineering efforts have succeeded to a certain extent in developing microbial strains that overproduce bioproducts at an industrial level. However, many metabolic engineering projects launched in academic labs do not reach commercial markets due to a failure to fully integrate industrial bioprocesses.
In response to this, Distinguished Professor Sang Yup Lee and Dr. Hyun Uk Kim, both from the Department of Chemical and Biomolecular Engineering at KAIST, have recently suggested ten general strategies of systems metabolic engineering to successfully develop industrial microbial strains. Systems metabolic engineering differs from conventional metabolic engineering by incorporating traditional metabolic engineering approaches along with tools of other fields, such as systems biology, synthetic biology, and molecular evolution.
The ten strategies of systems metabolic engineering have been featured in Nature Biotechnology released online in October 2015, which is entitled "Systems strategies for developing industrial microbial strains."
The strategies cover economic, state-of-the-art biological techniques and traditional bioprocess aspects. Specifically, they consist of: 1) project design including economic evaluation of a target bioproduct; 2) selection of host strains to be used for overproduction of a bioproduct; 3) metabolic pathway reconstruction for bioproducts that are not naturally produced in the selected host strains; 4) increasing tolerance of a host strain against the bioproduct; 5) removing negative regulatory circuits in the microbial host limiting overproduction of a bioproduct; 6) rerouting intracellular fluxes to optimize cofactor and precursor availability necessary for the bioproduct formation; 7) diagnosing and optimizing metabolic fluxes towards product formation; 8) diagnosis and optimization of microbial culture conditions including carbon sources; 9) system-wide gene manipulation to further increase the host strain's production performance using high-throughput genome-scale engineering and computational tools; and 10) scale-up fermentation of the developed strain and diagnosis for the reproducibility of the strain's production performance.
These ten strategies were articulated with successful examples of the production of L-arginine using Corynebacterium glutamicum, 1,4-butanediol using Escherichia coli, and L-lysine and bio-nylon using C. glutamicum.
Professor Sang Yup Lee said, "At the moment, the chance of commercializing microbial strains developed in academic labs is very low. The strategies of systems metabolic engineering outlined in this analysis can serve as guidelines when developing industrial microbial strains. We hope that these strategies contribute to improving opportunities to commercialize microbial strains developed in academic labs with drastically reduced costs and efforts, and that a large fraction of petroleum-based processes will be replaced with sustainable bioprocesses."
Lee S. Y. & Kim, H. U. Systems Strategies for Developing Industrial Microbial Strains. Nature Biotechnology (2015).
This work was supported by the Technology Development Program to Solve Climate Change on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556) and by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) from the Ministry of Science, ICT and Future Planning (MSIP), Korea, and through the National Research Foundation (NRF) of Korea. This work was also supported by the Novo Nordisk Foundation.
Picture: Concept of the Systems Metabolic Engineering Framework
(a) Three major bioprocess stages (b) Considerations in systems metabolic engineering to optimize the whole bioprocess. List of considerations for the strain development and fermentation contribute to improving microbial strain's production performance (red), whereas those for the separation and purification help in reducing overall operation costs by facilitating the downstream process (blue). Some of the considerations can be repeated in the course of systems metabolic engineering.
2015.10.19 View 11846
Establishment of System Metabolic Engineering Strategies
Although conventional petrochemical processes have generated chemicals and materials which have been useful to mankind, they have also triggered a variety of environmental problems including climate change and relied too much on nonrenewable natural resources. To ameliorate this, researchers have actively pursued the development of industrial microbial strains around the globe in order to overproduce industrially useful chemicals and materials from microbes using renewable biomass. This discipline is called metabolic engineering.
Thanks to advances in genetic engineering and our knowledge of cellular metabolism, conventional metabolic engineering efforts have succeeded to a certain extent in developing microbial strains that overproduce bioproducts at an industrial level. However, many metabolic engineering projects launched in academic labs do not reach commercial markets due to a failure to fully integrate industrial bioprocesses.
In response to this, Distinguished Professor Sang Yup Lee and Dr. Hyun Uk Kim, both from the Department of Chemical and Biomolecular Engineering at KAIST, have recently suggested ten general strategies of systems metabolic engineering to successfully develop industrial microbial strains. Systems metabolic engineering differs from conventional metabolic engineering by incorporating traditional metabolic engineering approaches along with tools of other fields, such as systems biology, synthetic biology, and molecular evolution.
The ten strategies of systems metabolic engineering have been featured in Nature Biotechnology released online in October 2015, which is entitled "Systems strategies for developing industrial microbial strains."
The strategies cover economic, state-of-the-art biological techniques and traditional bioprocess aspects. Specifically, they consist of: 1) project design including economic evaluation of a target bioproduct; 2) selection of host strains to be used for overproduction of a bioproduct; 3) metabolic pathway reconstruction for bioproducts that are not naturally produced in the selected host strains; 4) increasing tolerance of a host strain against the bioproduct; 5) removing negative regulatory circuits in the microbial host limiting overproduction of a bioproduct; 6) rerouting intracellular fluxes to optimize cofactor and precursor availability necessary for the bioproduct formation; 7) diagnosing and optimizing metabolic fluxes towards product formation; 8) diagnosis and optimization of microbial culture conditions including carbon sources; 9) system-wide gene manipulation to further increase the host strain's production performance using high-throughput genome-scale engineering and computational tools; and 10) scale-up fermentation of the developed strain and diagnosis for the reproducibility of the strain's production performance.
These ten strategies were articulated with successful examples of the production of L-arginine using Corynebacterium glutamicum, 1,4-butanediol using Escherichia coli, and L-lysine and bio-nylon using C. glutamicum.
Professor Sang Yup Lee said, "At the moment, the chance of commercializing microbial strains developed in academic labs is very low. The strategies of systems metabolic engineering outlined in this analysis can serve as guidelines when developing industrial microbial strains. We hope that these strategies contribute to improving opportunities to commercialize microbial strains developed in academic labs with drastically reduced costs and efforts, and that a large fraction of petroleum-based processes will be replaced with sustainable bioprocesses."
Lee S. Y. & Kim, H. U. Systems Strategies for Developing Industrial Microbial Strains. Nature Biotechnology (2015).
This work was supported by the Technology Development Program to Solve Climate Change on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556) and by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) from the Ministry of Science, ICT and Future Planning (MSIP), Korea, and through the National Research Foundation (NRF) of Korea. This work was also supported by the Novo Nordisk Foundation.
Picture: Concept of the Systems Metabolic Engineering Framework
(a) Three major bioprocess stages (b) Considerations in systems metabolic engineering to optimize the whole bioprocess. List of considerations for the strain development and fermentation contribute to improving microbial strain's production performance (red), whereas those for the separation and purification help in reducing overall operation costs by facilitating the downstream process (blue). Some of the considerations can be repeated in the course of systems metabolic engineering.
2015.10.19 View 11846 -
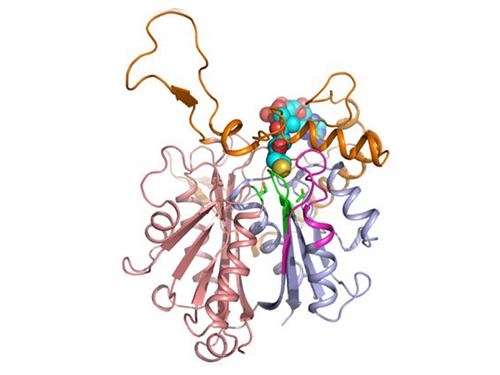 Discovery of Redox-Switch of KEenzyme Involved in N-Butanol Biosynthesis
Research teams at KAIST and Kyungpook National University (KNU) have succeeded in uncovering the redox-switch of thiolase, a key enzyme for n-butanol production in Clostridium acetobutylicum, one of the best known butanol-producing bacteria.
Biological n-butanol production was first reported by Louis Pasteur in 1861, and the bioprocess was industrialized usingClostridium acetobutylicum. The fermentation process by Clostridium strains has been known to be the most efficient one for n-butanol production. Due to growing world-wide issues such as energy security and climate change, the biological production of n-butanol has been receiving much renewed interest. This is because n-butanol possesses much better fuel characteristics compared to ethanol, such as higher energy content (29.2 MJ/L vs 19.6 MJ/L), less corrosiveness, less hygroscopy, and the ease with which it can be blended with gasoline and diesel.
In the paper published in Nature Communications, a broad-scope, online-only, and open access journal issued by the Nature Publishing Group (NPG), on September 22, 2015, Professor Kyung-Jin Kim at the School of Life Sciences, KNU, and Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering, KAIST, have proved that the redox-switch of thiolase plays a role in a regulation of metabolic flux in C. acetobutylicum by using in silico modeling and simulation tools.
The research team has redesigned thiolase with enhanced activity on the basis of the 3D structure of the wild-type enzyme. To reinforce a metabolic flux toward butanol production, the metabolic network of C. acetobutylicum strain was engineered with the redesigned enzyme. The combination of the discovery of 3D enzyme structure and systems metabolic engineering approaches resulted in increased n-butanol production in C. acetobutylicum, which allows the production of this important industrial chemical to be cost competitive.
Professors Kim and Lee said, "We have reported the 3D structure of C. acetobutylicum thiolase-a key enzyme involved in n-butanol biosynthesis, for the first time. Further study will be done to produce butanol more economically on the basis of the 3D structure of C. acetobutylicum thiolase."
This work was published online in Nature Communications on September 22, 2015.
Reference: Kim et al. "Redox-switch regulatory mechanism of thiolase from Clostridium acetobutylicum," Nature Communications
This research was supported by the Technology Development Program to Solve Climate Changes from the Ministry of Education, Science and Technology (MEST), Korea, the National Research Foundation of Korea, and the Advanced Biomass Center through the Global Frontier Research Program of the MEST, Korea.
For further information, contact Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930); and Dr. Kyung-Jin Kim, Professor, KNU, Daegu, Korea (kkim@knu.ac.kr, +82-53-950-6088).
Figure 1: A redox-switch of thiolase involves in butanol biosynthesis in Clostridium acetobutylicum. Thiolase condenses two acetyl-CoA molecules for initiating four carbon flux towards butanol.
Figure 2: Thiolase catalyzes the condensation reaction of acetyl-CoA to acetoacetyl-CoA. Two catalytic cysteine residues at 88th and 378th are oxidized and formed an intermolecular disulfide bond in an oxidized status, which results in inactivation of the enzyme for n-butanol biosynthesis. The intermolecular disulfide bond is broken enabling the n-butanol biosynthesis, when the environment status is reduced.
2015.09.23 View 11686
Discovery of Redox-Switch of KEenzyme Involved in N-Butanol Biosynthesis
Research teams at KAIST and Kyungpook National University (KNU) have succeeded in uncovering the redox-switch of thiolase, a key enzyme for n-butanol production in Clostridium acetobutylicum, one of the best known butanol-producing bacteria.
Biological n-butanol production was first reported by Louis Pasteur in 1861, and the bioprocess was industrialized usingClostridium acetobutylicum. The fermentation process by Clostridium strains has been known to be the most efficient one for n-butanol production. Due to growing world-wide issues such as energy security and climate change, the biological production of n-butanol has been receiving much renewed interest. This is because n-butanol possesses much better fuel characteristics compared to ethanol, such as higher energy content (29.2 MJ/L vs 19.6 MJ/L), less corrosiveness, less hygroscopy, and the ease with which it can be blended with gasoline and diesel.
In the paper published in Nature Communications, a broad-scope, online-only, and open access journal issued by the Nature Publishing Group (NPG), on September 22, 2015, Professor Kyung-Jin Kim at the School of Life Sciences, KNU, and Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering, KAIST, have proved that the redox-switch of thiolase plays a role in a regulation of metabolic flux in C. acetobutylicum by using in silico modeling and simulation tools.
The research team has redesigned thiolase with enhanced activity on the basis of the 3D structure of the wild-type enzyme. To reinforce a metabolic flux toward butanol production, the metabolic network of C. acetobutylicum strain was engineered with the redesigned enzyme. The combination of the discovery of 3D enzyme structure and systems metabolic engineering approaches resulted in increased n-butanol production in C. acetobutylicum, which allows the production of this important industrial chemical to be cost competitive.
Professors Kim and Lee said, "We have reported the 3D structure of C. acetobutylicum thiolase-a key enzyme involved in n-butanol biosynthesis, for the first time. Further study will be done to produce butanol more economically on the basis of the 3D structure of C. acetobutylicum thiolase."
This work was published online in Nature Communications on September 22, 2015.
Reference: Kim et al. "Redox-switch regulatory mechanism of thiolase from Clostridium acetobutylicum," Nature Communications
This research was supported by the Technology Development Program to Solve Climate Changes from the Ministry of Education, Science and Technology (MEST), Korea, the National Research Foundation of Korea, and the Advanced Biomass Center through the Global Frontier Research Program of the MEST, Korea.
For further information, contact Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930); and Dr. Kyung-Jin Kim, Professor, KNU, Daegu, Korea (kkim@knu.ac.kr, +82-53-950-6088).
Figure 1: A redox-switch of thiolase involves in butanol biosynthesis in Clostridium acetobutylicum. Thiolase condenses two acetyl-CoA molecules for initiating four carbon flux towards butanol.
Figure 2: Thiolase catalyzes the condensation reaction of acetyl-CoA to acetoacetyl-CoA. Two catalytic cysteine residues at 88th and 378th are oxidized and formed an intermolecular disulfide bond in an oxidized status, which results in inactivation of the enzyme for n-butanol biosynthesis. The intermolecular disulfide bond is broken enabling the n-butanol biosynthesis, when the environment status is reduced.
2015.09.23 View 11686