Atom
-
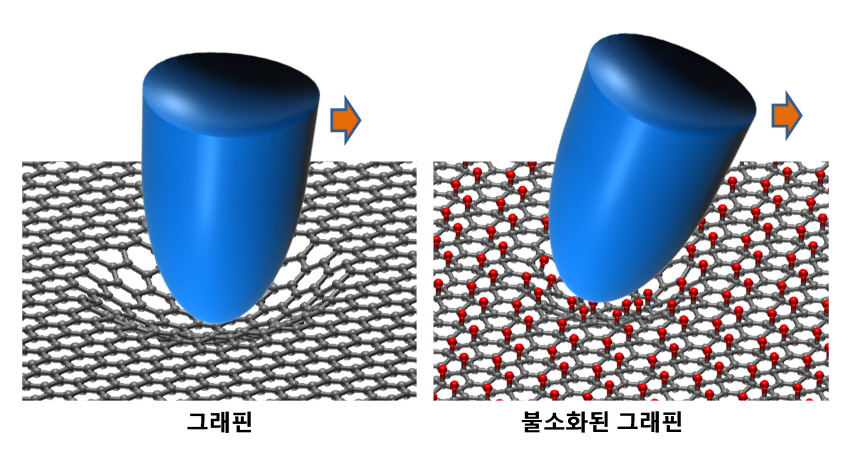 KAIST researchers verify and control the mechanical properties of graphene
KAIST researchers have successfully verified and controlled the mechanical properties of graphene, a next-generation material. Professor Park Jung Yong from the EEWS Graduate School and Professor Kim Yong Hyun from the Graduate School of Nanoscience and Technology have succeeded in fluorinating a single atomic-layered graphene sample and controlling its frictional and adhesive properties. This is the first time the frictional properties of graphene have been examined at the atomic level, and the technology is expected to be applied to nano-sized robots and microscopic joints.
Graphene is often dubbed “the dream material” because of its ability to conduct high amounts of electricity even when bent, making it the next-generation substitute for silicon semiconductors, paving the way for flexible display and wearable computer technologies. Graphene also has high potential applications in mechanical engineering because of its great material strength, but its mechanical properties remained elusive until now.
Professor Park’s research team successfully produced individual graphene samples with fluorine-deficiency at the atomic level by placing the samples in Fluoro-xenon (XeF2) gas and applying heat. The surface of the graphene was scanned using a micro probe and a high vacuum atomic microscope to measure its dynamic properties.
The research team found that the fluorinated graphene sample had 6 times more friction and 0.7 times more adhesiveness than the original graphene. Electrical measurements confirmed the fluorination process, and the analysis of the findings helped setup the theory of frictional changes in graphene.
Professor Park stated that “graphene can be used for the lubrication of joints in nano-sized devices” and that this research has numerous applications such as the coating of graphene-based microdynamic devices.
This research was published in the online June edition of Nano Letters and was supported by the Ministry of Science, Technology, and Education and the National Research Foundation as part of the World Class University (WCU) program.
2012.07.24 View 19359
KAIST researchers verify and control the mechanical properties of graphene
KAIST researchers have successfully verified and controlled the mechanical properties of graphene, a next-generation material. Professor Park Jung Yong from the EEWS Graduate School and Professor Kim Yong Hyun from the Graduate School of Nanoscience and Technology have succeeded in fluorinating a single atomic-layered graphene sample and controlling its frictional and adhesive properties. This is the first time the frictional properties of graphene have been examined at the atomic level, and the technology is expected to be applied to nano-sized robots and microscopic joints.
Graphene is often dubbed “the dream material” because of its ability to conduct high amounts of electricity even when bent, making it the next-generation substitute for silicon semiconductors, paving the way for flexible display and wearable computer technologies. Graphene also has high potential applications in mechanical engineering because of its great material strength, but its mechanical properties remained elusive until now.
Professor Park’s research team successfully produced individual graphene samples with fluorine-deficiency at the atomic level by placing the samples in Fluoro-xenon (XeF2) gas and applying heat. The surface of the graphene was scanned using a micro probe and a high vacuum atomic microscope to measure its dynamic properties.
The research team found that the fluorinated graphene sample had 6 times more friction and 0.7 times more adhesiveness than the original graphene. Electrical measurements confirmed the fluorination process, and the analysis of the findings helped setup the theory of frictional changes in graphene.
Professor Park stated that “graphene can be used for the lubrication of joints in nano-sized devices” and that this research has numerous applications such as the coating of graphene-based microdynamic devices.
This research was published in the online June edition of Nano Letters and was supported by the Ministry of Science, Technology, and Education and the National Research Foundation as part of the World Class University (WCU) program.
2012.07.24 View 19359 -
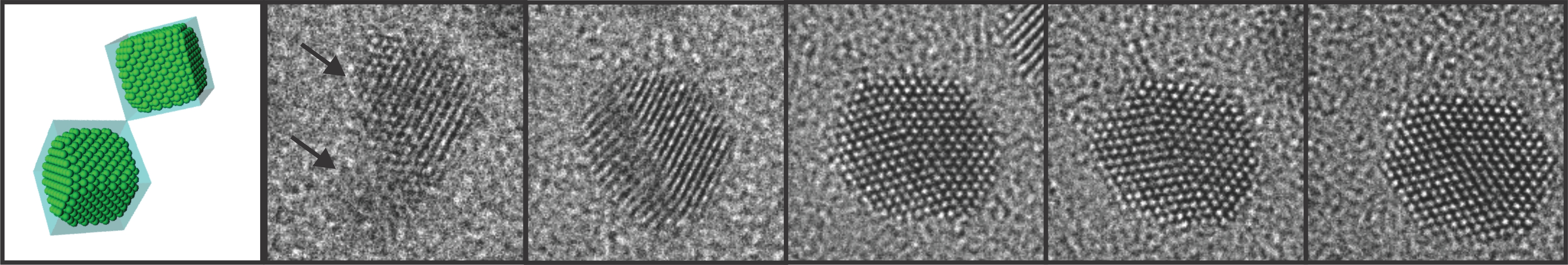 High-resolution Atomic Imaging of Specimens in Liquid Observed by Transmission Electron Microscopes Using Graphene Liquid Cells
Looking into specimens in liquid at the atomic level to understand nanoscale processes so far regarded as impossible to witnessThe Korea Advanced Institute of Science and Technology (KAIST) announced that a research team from the Department of Materials Science and Engineering has developed a technology that enables scientists and engineers to observe processes occurring in liquid media on the smallest possible scale which is less than a nanometer.
Professor Jeong Yong Lee and Researcher Jong Min Yuk, in collaboration with Professors Paul Alivisatos’s and Alex Zettl’s groups at the University of California, Berkeley, succeeded in making a graphene liquid cell or capsule, confining an ultra-thin liquid film between layers of graphene, for real-time and in situ imagining of nanoscale processes in fluids with atomic-level resolution by a transmission electron microscope (TEM). Their research was published in the April 6, 2012 issue of Science. (http://www.sciencemag.org/content/336/6077/61.abstract)
The graphene liquid cell (GLC) is composed of two sheets of graphene sandwiched to create a sealed chamber where a platinum growth solution is encapsulated in the form of a thin slice. Each graphene layer has a thickness of one carbon atom, the thinnest membrane that has ever been used to fabricate a liquid cell required for TEM.
The research team peered inside the GLC to observe the growth and dynamics of platinum nanocrystals in solution as they coalesced into a larger size, during which the graphene membrane with the encapsulated liquid remained intact. The researchers from KAIST and the UC Berkeley identified important features in the ongoing process of the nanocrystals’ coalescence and their expansion through coalescence to form certain shapes by imaging the phenomena with atomic-level resolution.
Professor Lee said,
“It has now become possible for scientists to observe what is happening in liquids on an atomic level under transmission electron microscopes.”
Researcher Yuk, one of the first authors of the paper, explained his research work.
“This research will promote other fields of study related to materials in a fluid stage including physical, chemical, and biological phenomena at the atomic level and promises numerous applications in the future. Pending further studies on liquid microscopy, the full application of a graphene-liquid-cell (GLC) TEM to biological samples is yet to be confirmed. Nonetheless, the GLC is the most effective technique developed today to sustain the natural state of fluid samples or species suspended in the liquid for a TEM imaging.”
The transmission electron microscope (TEM), first introduced in the 1930s, produces images at a significantly higher resolution than light microscopes, allowing users to examine the smallest level of physical, chemical, and biological phenomena. Observations by TEM with atomic resolution, however, have been limited to solid and/or frozen samples, and thus it has previously been impossible to study the real time fluid dynamics of liquid phases.
TEM imaging is performed in a high vacuum chamber in which a thin slice of the imaged sample is situated, and an electron beam passes through the slice to create an image. In this process, a liquid medium, unlike solid or frozen samples, evaporates, making it difficult to observe under TEM.
Attempts to produce a liquid capsule have thus far been made with electron-transparent membranes of such materials as silicon nitride or silicon oxide; such liquid capsules are relatively thick (tens to one hundred nanometers), however, resulting in poor electron transmittance with a reduced resolution of only a few nanometers. Silicon nitride is 25 nanometers thick, whereas graphene is only 0.34 nanometers.
Graphene, most commonly found in bulk graphite, is the thinnest material made out of carbon atoms. It has unique properties such as mechanical tensile strength, high flexibility, impermeability to small molecules, and high electrical conductivity. Graphene is an excellent material to hold micro- and nanoscopic objects for observation in a transmission electron microscope by minimizing scattering of the electron beam that irradiates a liquid sample while reducing charging and heating effects.
###
Figure 1. Schematic illustration of graphene liquid cells. Sandwiched two sheets of graphene encapsulate a platinum growth solution.
Figure 2. In-situ TEM observation of nanocrystal growth and shape evolution. TEM images of platinum nanocrystal coalescence and their faceting in the growth solution.
2012.04.23 View 13603
High-resolution Atomic Imaging of Specimens in Liquid Observed by Transmission Electron Microscopes Using Graphene Liquid Cells
Looking into specimens in liquid at the atomic level to understand nanoscale processes so far regarded as impossible to witnessThe Korea Advanced Institute of Science and Technology (KAIST) announced that a research team from the Department of Materials Science and Engineering has developed a technology that enables scientists and engineers to observe processes occurring in liquid media on the smallest possible scale which is less than a nanometer.
Professor Jeong Yong Lee and Researcher Jong Min Yuk, in collaboration with Professors Paul Alivisatos’s and Alex Zettl’s groups at the University of California, Berkeley, succeeded in making a graphene liquid cell or capsule, confining an ultra-thin liquid film between layers of graphene, for real-time and in situ imagining of nanoscale processes in fluids with atomic-level resolution by a transmission electron microscope (TEM). Their research was published in the April 6, 2012 issue of Science. (http://www.sciencemag.org/content/336/6077/61.abstract)
The graphene liquid cell (GLC) is composed of two sheets of graphene sandwiched to create a sealed chamber where a platinum growth solution is encapsulated in the form of a thin slice. Each graphene layer has a thickness of one carbon atom, the thinnest membrane that has ever been used to fabricate a liquid cell required for TEM.
The research team peered inside the GLC to observe the growth and dynamics of platinum nanocrystals in solution as they coalesced into a larger size, during which the graphene membrane with the encapsulated liquid remained intact. The researchers from KAIST and the UC Berkeley identified important features in the ongoing process of the nanocrystals’ coalescence and their expansion through coalescence to form certain shapes by imaging the phenomena with atomic-level resolution.
Professor Lee said,
“It has now become possible for scientists to observe what is happening in liquids on an atomic level under transmission electron microscopes.”
Researcher Yuk, one of the first authors of the paper, explained his research work.
“This research will promote other fields of study related to materials in a fluid stage including physical, chemical, and biological phenomena at the atomic level and promises numerous applications in the future. Pending further studies on liquid microscopy, the full application of a graphene-liquid-cell (GLC) TEM to biological samples is yet to be confirmed. Nonetheless, the GLC is the most effective technique developed today to sustain the natural state of fluid samples or species suspended in the liquid for a TEM imaging.”
The transmission electron microscope (TEM), first introduced in the 1930s, produces images at a significantly higher resolution than light microscopes, allowing users to examine the smallest level of physical, chemical, and biological phenomena. Observations by TEM with atomic resolution, however, have been limited to solid and/or frozen samples, and thus it has previously been impossible to study the real time fluid dynamics of liquid phases.
TEM imaging is performed in a high vacuum chamber in which a thin slice of the imaged sample is situated, and an electron beam passes through the slice to create an image. In this process, a liquid medium, unlike solid or frozen samples, evaporates, making it difficult to observe under TEM.
Attempts to produce a liquid capsule have thus far been made with electron-transparent membranes of such materials as silicon nitride or silicon oxide; such liquid capsules are relatively thick (tens to one hundred nanometers), however, resulting in poor electron transmittance with a reduced resolution of only a few nanometers. Silicon nitride is 25 nanometers thick, whereas graphene is only 0.34 nanometers.
Graphene, most commonly found in bulk graphite, is the thinnest material made out of carbon atoms. It has unique properties such as mechanical tensile strength, high flexibility, impermeability to small molecules, and high electrical conductivity. Graphene is an excellent material to hold micro- and nanoscopic objects for observation in a transmission electron microscope by minimizing scattering of the electron beam that irradiates a liquid sample while reducing charging and heating effects.
###
Figure 1. Schematic illustration of graphene liquid cells. Sandwiched two sheets of graphene encapsulate a platinum growth solution.
Figure 2. In-situ TEM observation of nanocrystal growth and shape evolution. TEM images of platinum nanocrystal coalescence and their faceting in the growth solution.
2012.04.23 View 13603 -
 New Era for Measuring Ultra Fast Phenomena: Atto Science Era
Domestic researchers successfully measured the exact status of the rapidly changing Helium atom using an atto second pulse. Thanks to this discovery, many ultrafast phenomena in nature can now be precisely measured. This will lead to an opening of a new "Atto Science" era.
Prof. Nam Chang Hee led this research team and Ph.d Kim Kyung Taek and Prof. Choi Nak Ryul also participated in this research. They have conducted the research under the support of the Researcher Support Program initiated by The Ministry of Education and Science and Korea Research Foundation. The research result was published in the prestigious journal "Physical Review Letters" on March 2nd. (Title: Amplitude and Phase Reconstruction of Electron Wave Packets for Probing Ultrafast Photoionization Dynamics)
Prof. Nam Chang Hee"s research team used atto second pulse to measure the ultrafast photoionization.
His team used atto second X-ray pulse and femto second laser pulse to photoionize Helium atoms, and measure the wave speed of the produced electron to closely investigate the ultrafast photoionization process.
Atom"s photoionization measurement using an atto second pulse was possible using the research team"s high-energy femto second laser and high-performance photo ion measurement device. This research team succeeded in producing the shortest 60 atto second pulse in the world using high-harmonic waves.
The research team used high-power femto second laser to produce atto second high-harmonic pulse from argon gas, used this to photoionize Helium atoms, and measured the ultrafast photoionization of the atoms.
Prof. Nam Chang Hee said, "This research precisely measured the exact status of rapidly changing Helium atoms. I am planning to research on measuring the ultrafast phenomena inside atoms and molecules and controlling the status of the atoms and molecules based on the research result."
2012.04.04 View 12696
New Era for Measuring Ultra Fast Phenomena: Atto Science Era
Domestic researchers successfully measured the exact status of the rapidly changing Helium atom using an atto second pulse. Thanks to this discovery, many ultrafast phenomena in nature can now be precisely measured. This will lead to an opening of a new "Atto Science" era.
Prof. Nam Chang Hee led this research team and Ph.d Kim Kyung Taek and Prof. Choi Nak Ryul also participated in this research. They have conducted the research under the support of the Researcher Support Program initiated by The Ministry of Education and Science and Korea Research Foundation. The research result was published in the prestigious journal "Physical Review Letters" on March 2nd. (Title: Amplitude and Phase Reconstruction of Electron Wave Packets for Probing Ultrafast Photoionization Dynamics)
Prof. Nam Chang Hee"s research team used atto second pulse to measure the ultrafast photoionization.
His team used atto second X-ray pulse and femto second laser pulse to photoionize Helium atoms, and measure the wave speed of the produced electron to closely investigate the ultrafast photoionization process.
Atom"s photoionization measurement using an atto second pulse was possible using the research team"s high-energy femto second laser and high-performance photo ion measurement device. This research team succeeded in producing the shortest 60 atto second pulse in the world using high-harmonic waves.
The research team used high-power femto second laser to produce atto second high-harmonic pulse from argon gas, used this to photoionize Helium atoms, and measured the ultrafast photoionization of the atoms.
Prof. Nam Chang Hee said, "This research precisely measured the exact status of rapidly changing Helium atoms. I am planning to research on measuring the ultrafast phenomena inside atoms and molecules and controlling the status of the atoms and molecules based on the research result."
2012.04.04 View 12696 -
 Quantum Mechanical Calculation Theory Developed
An Electron Density Functional Calculation Theory, based on the widely used quantum mechanical principles and yet accurate and with shortened calculation period, was developed by Korean research team.
*Electron Density Functional Calculation Theory: Theory that proves that it is possible to calculate energy and properties with only simple wave equations and electron densities.
The research was conducted by Professor Jeong Yoo Sung (Graduate School of EEWS) and Professor William Goddard with support from WCU Foster Project initiated by Ministry of Education, Science and Technology and Korea Research Foundation. The result was published in the Proceedings of the National Academy of Sciences Journal.
The research team corrected the error when performing quantum calculations that arises from the length of calculation time and incorrect assumptions and developed a theory and algorithm that is more accurate and faster. The use of wave equations in quantum mechanical calculations results in high accuracy but there is a rapid increase in calculation time and is therefore difficult to implement in large molecules with hundreds, or thousands of atoms. By implementing a low electron density variable with relatively less calculation work, the size of calculable molecule increases but the accuracy decreases.
The team focused on the interaction between electrons with different spins to improve upon the speed of calculation in the conventional accurate calculation. The team used the fact that the interaction between electrons with different spins increases as it comes closer together in accordance with the Pauli’s Exclusion Principle.
In addition the interaction between electrons are local and therefore can ignore the interactions between far away electrons and still get the total energy value. The team also took advantage of this fact and developed the algorithm that decreased calculation time hundredth fold.
Professor Jeong commented that, “So far most of the domestic achievements were made by focusing on integrative researches by calculation science and material design communities but these involved short time frames. In areas that required lengthy time frames like fundamentals and software development, there was no competitive advantage. However this research is significant in that a superior solution was developed domestically”.
2012.01.31 View 13937
Quantum Mechanical Calculation Theory Developed
An Electron Density Functional Calculation Theory, based on the widely used quantum mechanical principles and yet accurate and with shortened calculation period, was developed by Korean research team.
*Electron Density Functional Calculation Theory: Theory that proves that it is possible to calculate energy and properties with only simple wave equations and electron densities.
The research was conducted by Professor Jeong Yoo Sung (Graduate School of EEWS) and Professor William Goddard with support from WCU Foster Project initiated by Ministry of Education, Science and Technology and Korea Research Foundation. The result was published in the Proceedings of the National Academy of Sciences Journal.
The research team corrected the error when performing quantum calculations that arises from the length of calculation time and incorrect assumptions and developed a theory and algorithm that is more accurate and faster. The use of wave equations in quantum mechanical calculations results in high accuracy but there is a rapid increase in calculation time and is therefore difficult to implement in large molecules with hundreds, or thousands of atoms. By implementing a low electron density variable with relatively less calculation work, the size of calculable molecule increases but the accuracy decreases.
The team focused on the interaction between electrons with different spins to improve upon the speed of calculation in the conventional accurate calculation. The team used the fact that the interaction between electrons with different spins increases as it comes closer together in accordance with the Pauli’s Exclusion Principle.
In addition the interaction between electrons are local and therefore can ignore the interactions between far away electrons and still get the total energy value. The team also took advantage of this fact and developed the algorithm that decreased calculation time hundredth fold.
Professor Jeong commented that, “So far most of the domestic achievements were made by focusing on integrative researches by calculation science and material design communities but these involved short time frames. In areas that required lengthy time frames like fundamentals and software development, there was no competitive advantage. However this research is significant in that a superior solution was developed domestically”.
2012.01.31 View 13937 -
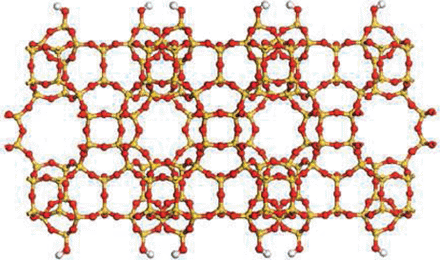 Ten Breakthroughs of the Year 2011 by Science
Porous Zeolite Crytals
Science, an internationally renowned scientific journal based in the US, has recently released a special issue of “Breakthrough of the Year, 2011,” dated December 23, 2011. In the issue, the journal introduces ten most important research breakthroughs made this year, and Professor Ryong Ryoo, Department of Chemistry at KAIST, was one of the scientists behind such notable advancements in 2011. Professor Ryoo has been highly regarded internationally for his research on the development of synthetic version of zeolites, a family of porous minerals that is widely used for products such as laundry detergents, cat litters, etc. Below is the article from Science, stating the zeolite research:
For Science’s “Breakthrough of the Year, 2011”, please go to:
http://www.sciencemag.org/site/special/btoy2011/
[Excerpt from the December 23, 2011 Issue of Science]
Industrial Molecules, Tailor-Made
If you ever doubt that chemistry is still a creative endeavor, just look at zeolites. This family of porous minerals was first discovered in 1756. They"re formed from different arrangements of aluminum, silicon, and oxygen atoms that crystallize into holey structures pocked with a perfect arrangement of pores. Over the past 250 years, 40 natural zeolites have been discovered, and chemists have chipped in roughly 150 more synthetic versions.
View larger version:
In this page
In a new window
Assembly required.
Porous zeolite crystals are widely used as filters and catalysts. This year, researchers found new ways to tailor the size of their pores and create thinner, cheaper membranes.
CREDIT: K. VAROON ET AL., SCIENCE334, 6052 (7 OCTOBER 2001)
This abundance isn"t just for show. Three million tons of zeolites are produced every year for use in laundry detergents, cat litter, and many other products. But zeolites really strut their stuff in two uses: as catalysts and molecular sieves. Oil refineries use zeolite catalysts to break down long hydrocarbon chains in oil into the shorter, volatile hydrocarbons in gasoline. And the minerals" small, regularly arranged pores make them ideal filters for purifying everything from the air on spaceships to the contaminated water around the nuclear reactors destroyed earlier this year in Fukushima, Japan.
Zeolites have their limitations, though. Their pores are almost universally tiny, making it tough to use them as catalysts for large molecules. And they"re difficult to form into ultrathin membranes, which researchers would like to do to enable cheaper separations. But progress by numerous teams on zeolite synthesis this year gave this “mature” area of chemistry new life.
Researchers in South Korea crafted a family of zeolites in which the usual network of small pores is surrounded by walls holed with larger voids. That combination of large and small pores should lead to catalysts for numerous large organic molecules.
Labs in Spain and China produced related large- and small-pore zeolites by using a combination of inorganic and organic materials to guide the structures as they formed.
Meanwhile, researchers in France and Germany discovered that, by carefully controlling growth conditions, they could form a large-pore zeolite without the need for the expensive organic compounds typically used to guide their architecture as they grow. The advance opens the way for cheaper catalysts. In yet another lab, researchers in Minnesota came up with a new route for making ultrathin zeolite membranes, which are likely to be useful as a wide variety of chemically selective filters.
This surge of molecular wizardry provides a vivid reminder that the creativity of chemists keeps their field ever young.
Related References and Web Sites
2011.12.23 View 14405
Ten Breakthroughs of the Year 2011 by Science
Porous Zeolite Crytals
Science, an internationally renowned scientific journal based in the US, has recently released a special issue of “Breakthrough of the Year, 2011,” dated December 23, 2011. In the issue, the journal introduces ten most important research breakthroughs made this year, and Professor Ryong Ryoo, Department of Chemistry at KAIST, was one of the scientists behind such notable advancements in 2011. Professor Ryoo has been highly regarded internationally for his research on the development of synthetic version of zeolites, a family of porous minerals that is widely used for products such as laundry detergents, cat litters, etc. Below is the article from Science, stating the zeolite research:
For Science’s “Breakthrough of the Year, 2011”, please go to:
http://www.sciencemag.org/site/special/btoy2011/
[Excerpt from the December 23, 2011 Issue of Science]
Industrial Molecules, Tailor-Made
If you ever doubt that chemistry is still a creative endeavor, just look at zeolites. This family of porous minerals was first discovered in 1756. They"re formed from different arrangements of aluminum, silicon, and oxygen atoms that crystallize into holey structures pocked with a perfect arrangement of pores. Over the past 250 years, 40 natural zeolites have been discovered, and chemists have chipped in roughly 150 more synthetic versions.
View larger version:
In this page
In a new window
Assembly required.
Porous zeolite crystals are widely used as filters and catalysts. This year, researchers found new ways to tailor the size of their pores and create thinner, cheaper membranes.
CREDIT: K. VAROON ET AL., SCIENCE334, 6052 (7 OCTOBER 2001)
This abundance isn"t just for show. Three million tons of zeolites are produced every year for use in laundry detergents, cat litter, and many other products. But zeolites really strut their stuff in two uses: as catalysts and molecular sieves. Oil refineries use zeolite catalysts to break down long hydrocarbon chains in oil into the shorter, volatile hydrocarbons in gasoline. And the minerals" small, regularly arranged pores make them ideal filters for purifying everything from the air on spaceships to the contaminated water around the nuclear reactors destroyed earlier this year in Fukushima, Japan.
Zeolites have their limitations, though. Their pores are almost universally tiny, making it tough to use them as catalysts for large molecules. And they"re difficult to form into ultrathin membranes, which researchers would like to do to enable cheaper separations. But progress by numerous teams on zeolite synthesis this year gave this “mature” area of chemistry new life.
Researchers in South Korea crafted a family of zeolites in which the usual network of small pores is surrounded by walls holed with larger voids. That combination of large and small pores should lead to catalysts for numerous large organic molecules.
Labs in Spain and China produced related large- and small-pore zeolites by using a combination of inorganic and organic materials to guide the structures as they formed.
Meanwhile, researchers in France and Germany discovered that, by carefully controlling growth conditions, they could form a large-pore zeolite without the need for the expensive organic compounds typically used to guide their architecture as they grow. The advance opens the way for cheaper catalysts. In yet another lab, researchers in Minnesota came up with a new route for making ultrathin zeolite membranes, which are likely to be useful as a wide variety of chemically selective filters.
This surge of molecular wizardry provides a vivid reminder that the creativity of chemists keeps their field ever young.
Related References and Web Sites
2011.12.23 View 14405 -
 Spintronics: A high wire act by Nanowerk News
An article by Nanowerk News on the integration of ferromagnetic nanowire arrays on grapheme substrates was published. Professor Bong-Soo Kim from the Department of Chemistry, KAIST, led the research in conjunction with Hanyang University and Samsung in Korea.
http://www.nanowerk.com/news/newsid=22204.php
Posted: Jul 25th, 2011
Spintronics: A high wire act
(Nanowerk News) Graphene is a promising material for a wide range of applications due to its remarkable mechanical and electronic properties. An application of particular interest is spin-based electronics, or spintronics, in which the spin orientation of an electron is used to perform circuit functions in addition to its charge. Bongsoo Kim and colleagues from KAIST, Hanyang University and Samsung in Korea now report the integration of ferromagnetic nanowire arrays on graphene substrates, opening up a route for the construction of graphene-based spintronic devices using nanowires as spin-injecting contacts ("Epitaxially Integrating Ferromagnetic Fe1.3Ge Nanowire Arrays on Few-Layer Graphene").
The spin of an electron is a property that, like charge, can be used to encode, process and transport information. However, spin information is easily lost in most media, which has made spintronics difficult to realize in practice. In graphene, on the other hand, spin can be preserved for longer due to its peculiar electron transport properties. "Low intrinsic spin–orbit coupling, long spin diffusion lengths and vanishing hyperfine interaction are features of graphene that make it a promising medium for spin transport," explains Kim.
Scanning electron microscopy image of vertical iron germanide nanowires grown on graphene. (© ACS 2011)
A prerequisite for the realization of spintronic devices based on graphene is its integration with ferromagnetic contacts to allow spin injection. Kim and his co-workers found that nanowires of iron germanide (Fe1.3Ge) serve as efficient contacts for this purpose. "Iron germanide nanowires show low resistivity and room-temperature ferromagnetism, and they are compatible with existing complementary metal–oxide–semiconductor technologies," says Kim.
To produce the atomically well-defined interfacial contact between the nanowires and the graphene surface needed for optimum device performance, the researchers deposited the contacts by an epitaxial method based on chemical vapor transport. Through careful adjustment of deposition parameters such as carrier gas flow rate and reaction temperature, the researchers produced vertically aligned nanowires that are closely lattice-matched to the graphene sheets (see image).
Initially preparing the graphene sheets on a substrate of silicon oxide allowed the researchers to isolate the final nanowire–graphene structure by etching and then transfer it to another substrate, greatly expanding the versatility of the approach. It is a delicate process, however. "It is necessary to transfer the graphene films onto the substrate very carefully in order to avoid folding and wrinkling of the graphene," says Kim.
Source: Tokyo Institute of Technology
2011.07.26 View 12302
Spintronics: A high wire act by Nanowerk News
An article by Nanowerk News on the integration of ferromagnetic nanowire arrays on grapheme substrates was published. Professor Bong-Soo Kim from the Department of Chemistry, KAIST, led the research in conjunction with Hanyang University and Samsung in Korea.
http://www.nanowerk.com/news/newsid=22204.php
Posted: Jul 25th, 2011
Spintronics: A high wire act
(Nanowerk News) Graphene is a promising material for a wide range of applications due to its remarkable mechanical and electronic properties. An application of particular interest is spin-based electronics, or spintronics, in which the spin orientation of an electron is used to perform circuit functions in addition to its charge. Bongsoo Kim and colleagues from KAIST, Hanyang University and Samsung in Korea now report the integration of ferromagnetic nanowire arrays on graphene substrates, opening up a route for the construction of graphene-based spintronic devices using nanowires as spin-injecting contacts ("Epitaxially Integrating Ferromagnetic Fe1.3Ge Nanowire Arrays on Few-Layer Graphene").
The spin of an electron is a property that, like charge, can be used to encode, process and transport information. However, spin information is easily lost in most media, which has made spintronics difficult to realize in practice. In graphene, on the other hand, spin can be preserved for longer due to its peculiar electron transport properties. "Low intrinsic spin–orbit coupling, long spin diffusion lengths and vanishing hyperfine interaction are features of graphene that make it a promising medium for spin transport," explains Kim.
Scanning electron microscopy image of vertical iron germanide nanowires grown on graphene. (© ACS 2011)
A prerequisite for the realization of spintronic devices based on graphene is its integration with ferromagnetic contacts to allow spin injection. Kim and his co-workers found that nanowires of iron germanide (Fe1.3Ge) serve as efficient contacts for this purpose. "Iron germanide nanowires show low resistivity and room-temperature ferromagnetism, and they are compatible with existing complementary metal–oxide–semiconductor technologies," says Kim.
To produce the atomically well-defined interfacial contact between the nanowires and the graphene surface needed for optimum device performance, the researchers deposited the contacts by an epitaxial method based on chemical vapor transport. Through careful adjustment of deposition parameters such as carrier gas flow rate and reaction temperature, the researchers produced vertically aligned nanowires that are closely lattice-matched to the graphene sheets (see image).
Initially preparing the graphene sheets on a substrate of silicon oxide allowed the researchers to isolate the final nanowire–graphene structure by etching and then transfer it to another substrate, greatly expanding the versatility of the approach. It is a delicate process, however. "It is necessary to transfer the graphene films onto the substrate very carefully in order to avoid folding and wrinkling of the graphene," says Kim.
Source: Tokyo Institute of Technology
2011.07.26 View 12302 -
 Dong Ah Newspaper Publish '100 Koreans who will Represent Korea in 10 years'
The 2011 list of ‘100 Koreans who will Represent Korea in 10 years’ published by Dong Ah Newspaper includes people of varying ages, vocation, and gender.
In terms of University Professors, five professors from each of KAIST and SNU (Seoul National University) were selected. Especially Professor Charles Ahn received the most votes due to his world class talent, potential, and dedication.
Professor Kim Sang Wook of the Department of Materials Science and Engineering is the world leading expert in the field of ‘Atom Construction Nanotechnology’ which deals with using macromolecules, carbon nanotubes, and grapheme to form various structures. His work on ‘low cost, large area nano patterning technology’ is expected to overcome the limits of nano treatment processes and its application in semi-conductors or displays carries great promise.
Professor Kim Eun Sung of the Department of Physics discovered a new quantum behavior, supersolidity, in a low temperature, solid Helium for the first time in the world and is the leading scientist that leads the mechanics behind such a phenomenon. Professor Kim is leading the field of supersolidity through his works on hidden phase in a low temperature solid Helium, the understanding the role of crystalline faults in the supersolidity phenomenon, and the destruction of the supersolid’s macromolecular phenomenon through spinning solids.
Professor Charles Ahn of the Graduate School of Innovation and Technology Management has been working as the developer of the V3 series (an anti-computer virus Vaccine Program) since 1988. He established the ‘Charles Ahn Research Center’ in 1995 and his solid and practical management style won him rave reviews. Professor Ahn was appointed as the Professor of the Graduate School of Innovation and Technology Management and has been teaching entrepreneurial perspective and Technology Management.
Professor Lee Sang Yeop of the Department of Biology and Chemical Engineering developed world’s most efficient production method of succinic acid, developed high efficiency, tailored, culture for the production of key amino acids, Valine and Threonine, developed the production culture off bio-buthanol which is superior to bio-ethanol, and is widely known as one of the leaders in the field of metabolic engineering.
Professor Jeong Ha Woong of the Department of Physics is being regarded as world leader in the field of Complex System Network Sciences. He implemented Statistical Physics to Complex Systems and also used the concept of ‘Networks’ and published 80 papers, including 5 which were published in Nature Magazine.
2011.04.30 View 15833
Dong Ah Newspaper Publish '100 Koreans who will Represent Korea in 10 years'
The 2011 list of ‘100 Koreans who will Represent Korea in 10 years’ published by Dong Ah Newspaper includes people of varying ages, vocation, and gender.
In terms of University Professors, five professors from each of KAIST and SNU (Seoul National University) were selected. Especially Professor Charles Ahn received the most votes due to his world class talent, potential, and dedication.
Professor Kim Sang Wook of the Department of Materials Science and Engineering is the world leading expert in the field of ‘Atom Construction Nanotechnology’ which deals with using macromolecules, carbon nanotubes, and grapheme to form various structures. His work on ‘low cost, large area nano patterning technology’ is expected to overcome the limits of nano treatment processes and its application in semi-conductors or displays carries great promise.
Professor Kim Eun Sung of the Department of Physics discovered a new quantum behavior, supersolidity, in a low temperature, solid Helium for the first time in the world and is the leading scientist that leads the mechanics behind such a phenomenon. Professor Kim is leading the field of supersolidity through his works on hidden phase in a low temperature solid Helium, the understanding the role of crystalline faults in the supersolidity phenomenon, and the destruction of the supersolid’s macromolecular phenomenon through spinning solids.
Professor Charles Ahn of the Graduate School of Innovation and Technology Management has been working as the developer of the V3 series (an anti-computer virus Vaccine Program) since 1988. He established the ‘Charles Ahn Research Center’ in 1995 and his solid and practical management style won him rave reviews. Professor Ahn was appointed as the Professor of the Graduate School of Innovation and Technology Management and has been teaching entrepreneurial perspective and Technology Management.
Professor Lee Sang Yeop of the Department of Biology and Chemical Engineering developed world’s most efficient production method of succinic acid, developed high efficiency, tailored, culture for the production of key amino acids, Valine and Threonine, developed the production culture off bio-buthanol which is superior to bio-ethanol, and is widely known as one of the leaders in the field of metabolic engineering.
Professor Jeong Ha Woong of the Department of Physics is being regarded as world leader in the field of Complex System Network Sciences. He implemented Statistical Physics to Complex Systems and also used the concept of ‘Networks’ and published 80 papers, including 5 which were published in Nature Magazine.
2011.04.30 View 15833 -
 Professor Min Beom Ki develops metamaterial with high index of refraction
Korean research team was able to theoretically prove that a metamaterial with high index of refraction does exist and produced it experimentally.
Professor Min Beom Ki, Dr. Choi Moo Han, and Doctorate candidate Lee Seung Hoon was joined by Dr. Kang Kwang Yong’s team from ETRI, KAIST’s Professor Less Yong Hee’s team, and Seoul National University’s Professor Park Nam Kyu’s team. The research was funded by the Basic Research Support Program initiated by the Ministry of Education, Science, and Technology and Korea Research Federation.
The result of the research was published in ‘Nature’ magazine and is one of the few researches carried out by teams composed entirely of Koreans.
Metamaterials are materials that have physical properties beyond those materials’ properties that are found in nature. It is formed not with atoms, but with synthetic atoms which have smaller structures than wavelengths.
The optical and electromagnetic waves’ properties of metamaterials can be altered significantly which has caught the attention of scientists worldwide.
Professor Min Beom Ki’s team independently designed and created a dielectric metamaterial with high polarization and low diamagnetism with an index of refraction of 38.6, highest synthesized index value.
It is expected that the result of the experiment will help develop high resolution imaging system and ultra small, hyper sensitive optical devices.
2011.02.23 View 21466
Professor Min Beom Ki develops metamaterial with high index of refraction
Korean research team was able to theoretically prove that a metamaterial with high index of refraction does exist and produced it experimentally.
Professor Min Beom Ki, Dr. Choi Moo Han, and Doctorate candidate Lee Seung Hoon was joined by Dr. Kang Kwang Yong’s team from ETRI, KAIST’s Professor Less Yong Hee’s team, and Seoul National University’s Professor Park Nam Kyu’s team. The research was funded by the Basic Research Support Program initiated by the Ministry of Education, Science, and Technology and Korea Research Federation.
The result of the research was published in ‘Nature’ magazine and is one of the few researches carried out by teams composed entirely of Koreans.
Metamaterials are materials that have physical properties beyond those materials’ properties that are found in nature. It is formed not with atoms, but with synthetic atoms which have smaller structures than wavelengths.
The optical and electromagnetic waves’ properties of metamaterials can be altered significantly which has caught the attention of scientists worldwide.
Professor Min Beom Ki’s team independently designed and created a dielectric metamaterial with high polarization and low diamagnetism with an index of refraction of 38.6, highest synthesized index value.
It is expected that the result of the experiment will help develop high resolution imaging system and ultra small, hyper sensitive optical devices.
2011.02.23 View 21466 -
 New Bio-Clock gene and its function found
The Ministry of Education, Science and Technology announced that a Korean research team has found a new gene responsible for maintaining the bio-clock (twenty-four) and its mechanism.
Twnety-four was led by Professor Choi Joon Ho and Dr. Lee Jong Bin of KAIST (department of Biology) and was a joint operation with Professor Ravi Allada and Dr.Lim Jeong Hoon of Northwestern University (department of neurobiology) and the result was published in ‘Nature’ magazine.
The research team experimented with transformed small fruit flies for 4 years and found that there was an undiscovered gene that deals with the bio rhythm in the brain which they named ‘twenty-four’.
The understanding with genes prior to twenty-four was that these genes regulate biorhythm in the transcription phase (DNA to mRNA). Twenty-four operates in the step after transcription when the ribosome creates proteins. Especially twenty-four has a great effect on the ‘period protein’ which acts as a sub-atomic clock that regulates the rhythm and life of each cell.
The experiment was innovational in that it was able to scientifically prove the function of the protein produced by the gene.
The result is expected to help solve the problems associated with sleep disorders, jetlags, eating rhythms, bio rhythms, etc.
The name twenty-four was the fact that a day, a cycle, is 24 hours long and the gene’s serial numbers CG4857 adds up to twenty four.
2011.02.23 View 15072
New Bio-Clock gene and its function found
The Ministry of Education, Science and Technology announced that a Korean research team has found a new gene responsible for maintaining the bio-clock (twenty-four) and its mechanism.
Twnety-four was led by Professor Choi Joon Ho and Dr. Lee Jong Bin of KAIST (department of Biology) and was a joint operation with Professor Ravi Allada and Dr.Lim Jeong Hoon of Northwestern University (department of neurobiology) and the result was published in ‘Nature’ magazine.
The research team experimented with transformed small fruit flies for 4 years and found that there was an undiscovered gene that deals with the bio rhythm in the brain which they named ‘twenty-four’.
The understanding with genes prior to twenty-four was that these genes regulate biorhythm in the transcription phase (DNA to mRNA). Twenty-four operates in the step after transcription when the ribosome creates proteins. Especially twenty-four has a great effect on the ‘period protein’ which acts as a sub-atomic clock that regulates the rhythm and life of each cell.
The experiment was innovational in that it was able to scientifically prove the function of the protein produced by the gene.
The result is expected to help solve the problems associated with sleep disorders, jetlags, eating rhythms, bio rhythms, etc.
The name twenty-four was the fact that a day, a cycle, is 24 hours long and the gene’s serial numbers CG4857 adds up to twenty four.
2011.02.23 View 15072 -
 The 6th president of KAIST passed away on May 7, 2010.
Dr. Sang-Soo Lee was the first president of Korea Advanced Institute of Science (KAIS) and the 6th president of KAIST, who died of a chronic disease at the age of 85. The KAIS was the matrix of KAIST today. Graduated from the physics department of Seoul National University in 1949, he later received a doctoral degree in optics from Imperial College of Science and Technology, University of London.
Dr. Lee has greatly contributed to the development of science and technology in Korea in the capacity of a policy administrator, educator, scientist, researcher, and engineer. He held numerous prestigious offices including President of Korea Atomic Energy Research Institute in 1967, of KAIS in 172, and of KAIST in 1989. Dr. Lee also worked as a professor at the physics department of KAIST for 20 years from 1972-1992.
The Society of Photographic Instrumentation Engineers (SPIE), an international society for optics and photonics, was founded in 1955 to advance light-based technologies. Dr. Sang-Soo Lee was a member of the SPIE that issued a news release expressing its sincere condolences to his death. The following is the full text of the news release: http://spie.org/x40527.xml
In memoriam: Sang Soo Lee
10 May 2010
Sang Soo Lee, known as the "Father of Optics" in Korea passed away on May 7, 2010, in Korea. He was 84.
Lee received a B.S. in Physics from Seoul National University in Korea and a Ph.D. from Imperial College of Science and Technology, University of London, UK. Receiving the first Ph.D. in Optics in Korea, Dr. Lee devoted his life to lay the foundation for optical science and engineering for more than four decades as an educator, researcher, and administrator in science policy.
"He was one of the architects of the extraordinary and rapid emergence of Korea as a world leader in science and technology, or perhaps with the rich history of contributions centuries ago, re-emergence would be more appropriate." said Eugene G. Arthurs, SPIE Executive Director.
During his teaching career, Dr. Lee mentored 50 doctoral and more than 100 masters" degree candidates. in the areas of laser physics, wave optics, and quantum optics. Many of his former students have become leaders in academia, government-funded research institutes, and industry both in Korea and abroad. He published more than 250 technical papers and authored five textbooks, including "Wave Optics", "Geometrical Optics", "Quantum Optics", and "Laser Speckles and Holography".
Lee was the first president of the Korea Advanced Institute of Science and Technology (KAIST), and the first president to establish a new government funded graduate school. He played a pivotal role in founding the Optical Society of Korea (OSK) in 1989 and served as its first president.
Lee was an active member of the international scientific community. In addition to his pioneering scholastic achievements at KAIST, he served as the Vice President of the International Commission for Optics (ICO), a Council Member of the Third World Academy of Sciences, and a Council Member of UN University, serving as an ambassador for the optics community, which showed a significant example of how a developing country like Korea can serve international optics community.
Dr. Lee was a Fellow of the International Society for Optical Engineering (SPIE), the Optical Society of America (OSA), and the Korean Physical Society (KPS). He was the recipient of many awards and honors, including the National Order of Civil Merit that is the Presidential Medal of Honor from the Republic of Korea (2000), the Songgok Academic Achievement Prize, the Presidential Award for Science, and the Medal of Honor for Distinguished Scientific Achievement in Korea. In 2006, he was awarded OSA"s Esther Hoffman Beller Medal.
2010.05.19 View 16885
The 6th president of KAIST passed away on May 7, 2010.
Dr. Sang-Soo Lee was the first president of Korea Advanced Institute of Science (KAIS) and the 6th president of KAIST, who died of a chronic disease at the age of 85. The KAIS was the matrix of KAIST today. Graduated from the physics department of Seoul National University in 1949, he later received a doctoral degree in optics from Imperial College of Science and Technology, University of London.
Dr. Lee has greatly contributed to the development of science and technology in Korea in the capacity of a policy administrator, educator, scientist, researcher, and engineer. He held numerous prestigious offices including President of Korea Atomic Energy Research Institute in 1967, of KAIS in 172, and of KAIST in 1989. Dr. Lee also worked as a professor at the physics department of KAIST for 20 years from 1972-1992.
The Society of Photographic Instrumentation Engineers (SPIE), an international society for optics and photonics, was founded in 1955 to advance light-based technologies. Dr. Sang-Soo Lee was a member of the SPIE that issued a news release expressing its sincere condolences to his death. The following is the full text of the news release: http://spie.org/x40527.xml
In memoriam: Sang Soo Lee
10 May 2010
Sang Soo Lee, known as the "Father of Optics" in Korea passed away on May 7, 2010, in Korea. He was 84.
Lee received a B.S. in Physics from Seoul National University in Korea and a Ph.D. from Imperial College of Science and Technology, University of London, UK. Receiving the first Ph.D. in Optics in Korea, Dr. Lee devoted his life to lay the foundation for optical science and engineering for more than four decades as an educator, researcher, and administrator in science policy.
"He was one of the architects of the extraordinary and rapid emergence of Korea as a world leader in science and technology, or perhaps with the rich history of contributions centuries ago, re-emergence would be more appropriate." said Eugene G. Arthurs, SPIE Executive Director.
During his teaching career, Dr. Lee mentored 50 doctoral and more than 100 masters" degree candidates. in the areas of laser physics, wave optics, and quantum optics. Many of his former students have become leaders in academia, government-funded research institutes, and industry both in Korea and abroad. He published more than 250 technical papers and authored five textbooks, including "Wave Optics", "Geometrical Optics", "Quantum Optics", and "Laser Speckles and Holography".
Lee was the first president of the Korea Advanced Institute of Science and Technology (KAIST), and the first president to establish a new government funded graduate school. He played a pivotal role in founding the Optical Society of Korea (OSK) in 1989 and served as its first president.
Lee was an active member of the international scientific community. In addition to his pioneering scholastic achievements at KAIST, he served as the Vice President of the International Commission for Optics (ICO), a Council Member of the Third World Academy of Sciences, and a Council Member of UN University, serving as an ambassador for the optics community, which showed a significant example of how a developing country like Korea can serve international optics community.
Dr. Lee was a Fellow of the International Society for Optical Engineering (SPIE), the Optical Society of America (OSA), and the Korean Physical Society (KPS). He was the recipient of many awards and honors, including the National Order of Civil Merit that is the Presidential Medal of Honor from the Republic of Korea (2000), the Songgok Academic Achievement Prize, the Presidential Award for Science, and the Medal of Honor for Distinguished Scientific Achievement in Korea. In 2006, he was awarded OSA"s Esther Hoffman Beller Medal.
2010.05.19 View 16885 -
 New Text Book on Chemistry Published by KAIST Professor and Student
A chemistry textbook written in English and Korean will aid Korean students to learn General Chemistry in a global academic setting.
Korean students majoring in chemistry and looking for an opportunity to study abroad will have a new, handy textbook that presents them with a practical introduction to an English speaking lecture on general chemistry.
Aiming for advanced Korean high school and college/university students, the inter-language textbook is written by two incumbent professors teaching chemistry at a university in Korea and the US. The book will help Korean students prepare for a classroom where various topics of general chemistry are presented and discussed in English. Clear, collated sections of English and Korean text provide the student with sufficient explanation of the rudimentary topics and concepts.
Composed of 15 chapters on the core subjects of General Chemistry, i.e., Stoichiometry and Chemical Reactions, Thermochemistry, Atomic Structure, and Bonding, the textbook includes essential English vocabulary and usage sections for each chapter; it also contains a pre-reading study guide on the subject that prepares the student for listening to a lecture. This section includes view-graph type slides, audio files, and follow-up questions the student can use to prepare for an English-speaking course. The various accompanying audio files are prepared to expose the student to English scientific dialogue and serve as examples for instruction at Korean secondary and tertiary schools.
The book was coauthored by Korean and American scientists: A father and son, who have taught chemistry at an American and Korean university, wrote the book. Professor Melvyn R. Churchill at the State University of New York at Buffalo and Professor David G. Churchill at KAIST prepared all of the technical English text which was adapted from General Chemistry course lecture notes; the text was further shaped by original perspectives arising from many student interactions and questions.
This English text was translated into Korean by Professor Kwanhee Lee from the Department of Life and Food Science at Handong Global University, who coauthored a previous preparatory book for Korean students in a different subject. He also supplied an important introductory section which serves as a general guide to the classroom student. Kibong Kim, a doctoral student in the Department of Chemistry at KAIST, helped in preparing the book as well.
“This has been definitely a collaborative undertaking with an international academic crew and it underscores that the Korean internationalization in science is mainstream. Professors and a Korean student created a new book for Korean consumption and benefit,” Professor David G. Churchill says.
----------------------------------------------------------------------------------------
Bibliography: “How to Prepare for General Chemistry Taught in English” by David George Churchill, Melvyn Rowen Churchill, Kwanhee Lee & Kibong Kim, Darakwon Publishing, Paju, Republic of Korea, 2010, 400 pp, ISBN 978-89-5995-730-9 (1 Audio CD included)
2010.04.02 View 18063
New Text Book on Chemistry Published by KAIST Professor and Student
A chemistry textbook written in English and Korean will aid Korean students to learn General Chemistry in a global academic setting.
Korean students majoring in chemistry and looking for an opportunity to study abroad will have a new, handy textbook that presents them with a practical introduction to an English speaking lecture on general chemistry.
Aiming for advanced Korean high school and college/university students, the inter-language textbook is written by two incumbent professors teaching chemistry at a university in Korea and the US. The book will help Korean students prepare for a classroom where various topics of general chemistry are presented and discussed in English. Clear, collated sections of English and Korean text provide the student with sufficient explanation of the rudimentary topics and concepts.
Composed of 15 chapters on the core subjects of General Chemistry, i.e., Stoichiometry and Chemical Reactions, Thermochemistry, Atomic Structure, and Bonding, the textbook includes essential English vocabulary and usage sections for each chapter; it also contains a pre-reading study guide on the subject that prepares the student for listening to a lecture. This section includes view-graph type slides, audio files, and follow-up questions the student can use to prepare for an English-speaking course. The various accompanying audio files are prepared to expose the student to English scientific dialogue and serve as examples for instruction at Korean secondary and tertiary schools.
The book was coauthored by Korean and American scientists: A father and son, who have taught chemistry at an American and Korean university, wrote the book. Professor Melvyn R. Churchill at the State University of New York at Buffalo and Professor David G. Churchill at KAIST prepared all of the technical English text which was adapted from General Chemistry course lecture notes; the text was further shaped by original perspectives arising from many student interactions and questions.
This English text was translated into Korean by Professor Kwanhee Lee from the Department of Life and Food Science at Handong Global University, who coauthored a previous preparatory book for Korean students in a different subject. He also supplied an important introductory section which serves as a general guide to the classroom student. Kibong Kim, a doctoral student in the Department of Chemistry at KAIST, helped in preparing the book as well.
“This has been definitely a collaborative undertaking with an international academic crew and it underscores that the Korean internationalization in science is mainstream. Professors and a Korean student created a new book for Korean consumption and benefit,” Professor David G. Churchill says.
----------------------------------------------------------------------------------------
Bibliography: “How to Prepare for General Chemistry Taught in English” by David George Churchill, Melvyn Rowen Churchill, Kwanhee Lee & Kibong Kim, Darakwon Publishing, Paju, Republic of Korea, 2010, 400 pp, ISBN 978-89-5995-730-9 (1 Audio CD included)
2010.04.02 View 18063 -
 KAIST's OLEV Best Model of Creative Growth Engine
Various models of electric vehicles designed to replace the internal combustion automobiles face significant problems as they invariably failed to overcome the limitations involving lithium battery in terms of power capacity, weight, raw materal price, recharging time and preparation of charging stations. Worst of all, the limited supply of lithium will eventually raise its price sky high when all cars use lithium batteries, and the economic value of electric cars will be lost.
KAIST"s online electric vehicle project (OLEV) seeks to resolve these fundamental problems involving electric vehicles that have so far been developed. KAIST OLEV, a project to develop a new growth engine for the nation and lead the future of global automotive industry, is an entirely new concept: the electric vehicle picks up power from underground power supplier lines through the non-contact magnetic charging method, while either running or standing. This is the first eco-friendly and economic automotive system that can resolve the problems inherent to previously-developed electric vehicles, according to the KAIST OLEV Project Center.
In February 2009, KAIST researchers first proved that up to 80 percent power conveyance is possible through a gap of 1 centimeter from the power line, and in July they successfully supplied power to a bus -- up to 60 percent across a 12 cm gap from the power line embedded in the ground -- using power supply and pick-up devices they developed. In this process, KAIST has secured the core technologies for maximizing power efficiency and minimizing the cost of installing the non-contact power supply system. KAIST has established the Online Electric Vehicle Co., Ltd., to undertake business activities related to the OLEV project, including the IPR on power supply and pick-up devices, parts and accessories and commercial promotion. A demonstration event is scheduled for Aug. 13, Thursday.
The impact of the development of the OLEV technology on the energy and environment issues and the overall economy will be enormous. In case a half of the total automobiles running in Korea, or 6 million vehicles, are replaced with OLEV, electric power produced by just two of the nation"s atomic power plants will be enough to operate them all, and the nation will be able to reduce crude oil import by 35 million barrels worth U.S.$3 billion a year (supposing $80 per barrel).
Korea"s export of OLEV units will in the future surpass the present level of overseas sale of conventional cars. When nations use online electric vehicles in large numbers, their demand for CO2-free power plants will grow. Korea has cutting-edge technology in the construction of atomic power plants. As a world leader in the area of nuclear power plant, Korea will enjoy new opportunities to contribute to the global advancement of atomic power generation as well as transportation industries.
Korea still shares a small portion of the world"s automobile market estimated to worth some 2,000 trillion Korean won. But commercialization of the OLEV technology worldwide will greatly enhance Korea"s global automotive market share. Successful development of the online electric vehicle requires preemptive investment and positive support by the government for the ultimate purpose of resolving energy and environment problems.
If and when domestic enterprises secure technological supremacy in the next generation automobile market with their online electric vehicles which will replace the 100-year-old combustion engine, it will be the most desirable shortcut to raising Korea"s international competitiveness. OLEV promises to be the model of creative growth engine in the 21st century.
2009.07.30 View 19886
KAIST's OLEV Best Model of Creative Growth Engine
Various models of electric vehicles designed to replace the internal combustion automobiles face significant problems as they invariably failed to overcome the limitations involving lithium battery in terms of power capacity, weight, raw materal price, recharging time and preparation of charging stations. Worst of all, the limited supply of lithium will eventually raise its price sky high when all cars use lithium batteries, and the economic value of electric cars will be lost.
KAIST"s online electric vehicle project (OLEV) seeks to resolve these fundamental problems involving electric vehicles that have so far been developed. KAIST OLEV, a project to develop a new growth engine for the nation and lead the future of global automotive industry, is an entirely new concept: the electric vehicle picks up power from underground power supplier lines through the non-contact magnetic charging method, while either running or standing. This is the first eco-friendly and economic automotive system that can resolve the problems inherent to previously-developed electric vehicles, according to the KAIST OLEV Project Center.
In February 2009, KAIST researchers first proved that up to 80 percent power conveyance is possible through a gap of 1 centimeter from the power line, and in July they successfully supplied power to a bus -- up to 60 percent across a 12 cm gap from the power line embedded in the ground -- using power supply and pick-up devices they developed. In this process, KAIST has secured the core technologies for maximizing power efficiency and minimizing the cost of installing the non-contact power supply system. KAIST has established the Online Electric Vehicle Co., Ltd., to undertake business activities related to the OLEV project, including the IPR on power supply and pick-up devices, parts and accessories and commercial promotion. A demonstration event is scheduled for Aug. 13, Thursday.
The impact of the development of the OLEV technology on the energy and environment issues and the overall economy will be enormous. In case a half of the total automobiles running in Korea, or 6 million vehicles, are replaced with OLEV, electric power produced by just two of the nation"s atomic power plants will be enough to operate them all, and the nation will be able to reduce crude oil import by 35 million barrels worth U.S.$3 billion a year (supposing $80 per barrel).
Korea"s export of OLEV units will in the future surpass the present level of overseas sale of conventional cars. When nations use online electric vehicles in large numbers, their demand for CO2-free power plants will grow. Korea has cutting-edge technology in the construction of atomic power plants. As a world leader in the area of nuclear power plant, Korea will enjoy new opportunities to contribute to the global advancement of atomic power generation as well as transportation industries.
Korea still shares a small portion of the world"s automobile market estimated to worth some 2,000 trillion Korean won. But commercialization of the OLEV technology worldwide will greatly enhance Korea"s global automotive market share. Successful development of the online electric vehicle requires preemptive investment and positive support by the government for the ultimate purpose of resolving energy and environment problems.
If and when domestic enterprises secure technological supremacy in the next generation automobile market with their online electric vehicles which will replace the 100-year-old combustion engine, it will be the most desirable shortcut to raising Korea"s international competitiveness. OLEV promises to be the model of creative growth engine in the 21st century.
2009.07.30 View 19886