NT
-
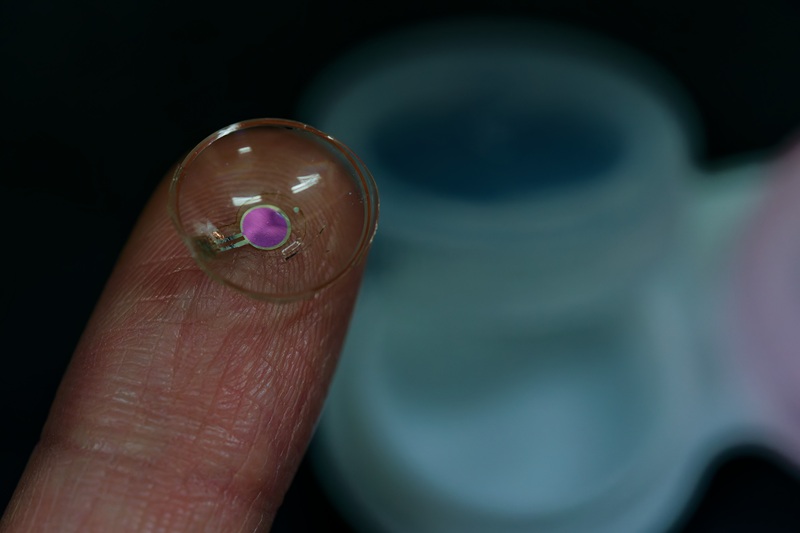 KAIST Develops World’s First Wireless OLED Contact Lens for Retinal Diagnostics
<ID-style photograph against a laboratory background featuring an OLED contact lens sample (center), flanked by the principal authors (left: Professor Seunghyup Yoo ; right: Dr. Jee Hoon Sim). Above them (from top to bottom) are: Professor Se Joon Woo, Professor Sei Kwang Hahn, Dr. Su-Bon Kim, and Dr. Hyeonwook Chae>
Electroretinography (ERG) is an ophthalmic diagnostic method used to determine whether the retina is functioning normally. It is widely employed for diagnosing hereditary retinal diseases or assessing retinal function decline.
A team of Korean researchers has developed a next-generation wireless ophthalmic diagnostic technology that replaces the existing stationary, darkroom-based retinal testing method by incorporating an “ultrathin OLED” into a contact lens. This breakthrough is expected to have applications in diverse fields such as myopia treatment, ocular biosignal analysis, augmented-reality (AR) visual information delivery, and light-based neurostimulation.
On the 12th, KAIST (President Kwang Hyung Lee) announced that a research team led by Professor Seunghyup Yoo from the School of Electrical Engineering, in collaboration with Professor Se Joon Woo of Seoul National University Bundang Hospital (Director Jeong-Han Song), Professor Sei Kwang Hahn of POSTECH (President Sung-Keun Kim) and CEO of PHI Biomed Co., and the Electronics and Telecommunications Research Institute (ETRI, President Seungchan Bang) under the National Research Council of Science & Technology (NST, Chairman Youngshik Kim), has developed the world’s first wireless contact lens-based wearable retinal diagnostic platform using organic light-emitting diodes (OLEDs).
<Figure 1. Schematic and photograph of the wireless OLED contact lens>
This technology enables ERG simply by wearing the lens, eliminating the need for large specialized light sources and dramatically simplifying the conventional, complex ophthalmic diagnostic environment.
Traditionally, ERG requires the use of a stationary Ganzfeld device in a dark room, where patients must keep their eyes open and remain still during the test. This setup imposes spatial constraints and can lead to patient fatigue and compliances challenges.
To overcome these limitations, the joint research team integrated an ultrathin flexible OLED —approximately 12.5 μm thick, or 6–8 times thinner than a human hair— into a contact lens electrode for ERG. They also equipped it with a wireless power receiving antenna and a control chip, completing a system capable of independent operation.
For power transmission, the team adopted a wireless power transfer method using a 433 MHz resonant frequency suitable for stable wireless communication. This was also demonstrated in the form of a wireless controller embedded in a sleep mask, which can be linked to a smartphone —further enhancing practical usability.
<Figure 2. Schematic of the electroretinography (ERG) testing system using a wireless OLED contact lens and an example of an actual test in progress>
While most smart contact lens–type light sources developed for ocular illumination have used inorganic LEDs, these rigid devices emit light almost from a single point, which can lead to excessive heat accumulation and thus usable light intensity. In contrast, OLEDs are areal light sources and were shown to induce retinal responses even under low luminance conditions. In this study, under a relatively low luminance* of 126 nits, the OLED contact lens successfully induced stable ERG signals, producing diagnostic results equivalent to those obtained with existing commercial light sources.
*Luminance: A value indicating how brightly a surface or screen emits light; for reference, the luminance of a smartphone screen is about 300–600 nits (can exceed 1000 nits at maximum).
Animal tests confirmed that the surface temperature of a rabbit’s eye wearing the OLED contact lens remained below 27°C, avoiding corneal heat damage, and that the light-emitting performance was maintained even in humid environments—demonstrating its effectiveness and safety as an ERG diagnostic tool in real clinical settings.
Professor Seunghyup Yoo stated that “integrating the flexibility and diffusive light characteristics of ultrathin OLEDs into a contact lens is a world-first attempt,” and that “this research can help expand smart contact lens technology into on-eye optical diagnostic and phototherapeutic platforms, contributing to the advancement of digital healthcare technology.”
< Wireless operation of the OLED contact lens >
Jee Hoon Sim, Hyeonwook Chae, and Su-Bon Kim, PhD researchers at KAIST, played a key role as co-first authors alongside Dr. Sangbaie Shin of PHI Biomed Co.. Corresponding authors are Professor Seunghyup Yoo (School of Electrical Engineering, KAIST), Professor Sei Kwang Hahn (Department of Materials Science and Engineering, POSTECH), and Professor Se Joon Woo (Seoul National University Bundang Hospital). The results were published online in the internationally renowned journal ACS Nano on May 1st.
● Paper title: Wireless Organic Light-Emitting Diode Contact Lenses for On-Eye Wearable Light Sources and Their Application to Personalized Health Monitoring
● DOI: https://doi.org/10.1021/acsnano.4c18563
● Related video clip: http://bit.ly/3UGg6R8
< Close-up of the OLED contact lens sample >
2025.08.12 View 748
KAIST Develops World’s First Wireless OLED Contact Lens for Retinal Diagnostics
<ID-style photograph against a laboratory background featuring an OLED contact lens sample (center), flanked by the principal authors (left: Professor Seunghyup Yoo ; right: Dr. Jee Hoon Sim). Above them (from top to bottom) are: Professor Se Joon Woo, Professor Sei Kwang Hahn, Dr. Su-Bon Kim, and Dr. Hyeonwook Chae>
Electroretinography (ERG) is an ophthalmic diagnostic method used to determine whether the retina is functioning normally. It is widely employed for diagnosing hereditary retinal diseases or assessing retinal function decline.
A team of Korean researchers has developed a next-generation wireless ophthalmic diagnostic technology that replaces the existing stationary, darkroom-based retinal testing method by incorporating an “ultrathin OLED” into a contact lens. This breakthrough is expected to have applications in diverse fields such as myopia treatment, ocular biosignal analysis, augmented-reality (AR) visual information delivery, and light-based neurostimulation.
On the 12th, KAIST (President Kwang Hyung Lee) announced that a research team led by Professor Seunghyup Yoo from the School of Electrical Engineering, in collaboration with Professor Se Joon Woo of Seoul National University Bundang Hospital (Director Jeong-Han Song), Professor Sei Kwang Hahn of POSTECH (President Sung-Keun Kim) and CEO of PHI Biomed Co., and the Electronics and Telecommunications Research Institute (ETRI, President Seungchan Bang) under the National Research Council of Science & Technology (NST, Chairman Youngshik Kim), has developed the world’s first wireless contact lens-based wearable retinal diagnostic platform using organic light-emitting diodes (OLEDs).
<Figure 1. Schematic and photograph of the wireless OLED contact lens>
This technology enables ERG simply by wearing the lens, eliminating the need for large specialized light sources and dramatically simplifying the conventional, complex ophthalmic diagnostic environment.
Traditionally, ERG requires the use of a stationary Ganzfeld device in a dark room, where patients must keep their eyes open and remain still during the test. This setup imposes spatial constraints and can lead to patient fatigue and compliances challenges.
To overcome these limitations, the joint research team integrated an ultrathin flexible OLED —approximately 12.5 μm thick, or 6–8 times thinner than a human hair— into a contact lens electrode for ERG. They also equipped it with a wireless power receiving antenna and a control chip, completing a system capable of independent operation.
For power transmission, the team adopted a wireless power transfer method using a 433 MHz resonant frequency suitable for stable wireless communication. This was also demonstrated in the form of a wireless controller embedded in a sleep mask, which can be linked to a smartphone —further enhancing practical usability.
<Figure 2. Schematic of the electroretinography (ERG) testing system using a wireless OLED contact lens and an example of an actual test in progress>
While most smart contact lens–type light sources developed for ocular illumination have used inorganic LEDs, these rigid devices emit light almost from a single point, which can lead to excessive heat accumulation and thus usable light intensity. In contrast, OLEDs are areal light sources and were shown to induce retinal responses even under low luminance conditions. In this study, under a relatively low luminance* of 126 nits, the OLED contact lens successfully induced stable ERG signals, producing diagnostic results equivalent to those obtained with existing commercial light sources.
*Luminance: A value indicating how brightly a surface or screen emits light; for reference, the luminance of a smartphone screen is about 300–600 nits (can exceed 1000 nits at maximum).
Animal tests confirmed that the surface temperature of a rabbit’s eye wearing the OLED contact lens remained below 27°C, avoiding corneal heat damage, and that the light-emitting performance was maintained even in humid environments—demonstrating its effectiveness and safety as an ERG diagnostic tool in real clinical settings.
Professor Seunghyup Yoo stated that “integrating the flexibility and diffusive light characteristics of ultrathin OLEDs into a contact lens is a world-first attempt,” and that “this research can help expand smart contact lens technology into on-eye optical diagnostic and phototherapeutic platforms, contributing to the advancement of digital healthcare technology.”
< Wireless operation of the OLED contact lens >
Jee Hoon Sim, Hyeonwook Chae, and Su-Bon Kim, PhD researchers at KAIST, played a key role as co-first authors alongside Dr. Sangbaie Shin of PHI Biomed Co.. Corresponding authors are Professor Seunghyup Yoo (School of Electrical Engineering, KAIST), Professor Sei Kwang Hahn (Department of Materials Science and Engineering, POSTECH), and Professor Se Joon Woo (Seoul National University Bundang Hospital). The results were published online in the internationally renowned journal ACS Nano on May 1st.
● Paper title: Wireless Organic Light-Emitting Diode Contact Lenses for On-Eye Wearable Light Sources and Their Application to Personalized Health Monitoring
● DOI: https://doi.org/10.1021/acsnano.4c18563
● Related video clip: http://bit.ly/3UGg6R8
< Close-up of the OLED contact lens sample >
2025.08.12 View 748 -
 KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo >
Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model that, using only information about the target protein, can design optimal drug candidates without any prior molecular data—opening up new possibilities for drug discovery.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Professor Woo Youn Kim in the Department of Chemistry has developed an AI model named BInD (Bond and Interaction-generating Diffusion model), which can design and optimize drug candidate molecules tailored to a protein’s structure alone—without needing prior information about binding molecules. The model also predicts the binding mechanism (non-covalent interactions) between the drug and the target protein.
The core innovation of this technology lies in its “simultaneous design” approach. Previous AI models either focused on generating molecules or separately evaluating whether the generated molecule could bind to the target protein. In contrast, this new model considers the binding mechanism between the molecule and the protein during the generation process, enabling comprehensive design in one step. Since it pre-accounts for critical factors in protein-ligand binding, it has a much higher likelihood of generating effective and stable molecules. The generation process visually demonstrates how types and positions of atoms, covalent bonds, and interactions are created simultaneously to fit the protein’s binding site.
<Figure 1. Schematic of the diffusion model developed by the research team, which generates molecular structures and non-covalent interactions based on protein structures. Starting from a noise distribution, the model gradually removes noise (via reverse diffusion) to restore the atom positions, types, covalent bond types, and interaction types, thereby generating molecules. Interacting patterns are extracted from prior knowledge of known binding molecules or proteins, and through an inpainting technique, these patterns are kept fixed during the reverse diffusion process to guide the molecular generation.>
Moreover, this model is designed to meet multiple essential drug design criteria simultaneously—such as target binding affinity, drug-like properties, and structural stability. Traditional models often optimized for only one or two goals at the expense of others, but this new model balances various objectives, significantly enhancing its practical applicability.
The research team explained that the AI operates based on a “diffusion model”—a generative approach where a structure becomes increasingly refined from a random state. This is the same type of model used in AlphaFold 3, the 2024 Nobel Chemistry Prize-winning tool for protein-ligand structure generation, which has already demonstrated high efficiency.
Unlike AlphaFold 3, which provides spatial coordinates for atom positions, this study introduced a knowledge-based guide grounded in actual chemical laws—such as bond lengths and protein-ligand distances—enabling more chemically realistic structure generation.
<Figure 2. (Left) Target protein and the original bound molecule; (Right) Examples of molecules designed using the model developed in this study. The values for protein binding affinity (Vina), drug-likeness (QED), and synthetic accessibility (SA) are shown at the bottom.>
Additionally, the team applied an optimization strategy where outstanding binding patterns from prior results are reused. This allowed the model to generate even better drug candidates without additional training. Notably, the AI successfully produced molecules that selectively bind to the mutated residues of EGFR, a cancer-related target protein.
This study is also meaningful because it advances beyond the team’s previous research, which required prior input about the molecular conditions for the interaction pattern of protein binding.
Professor Woo Youn Kim commented that “the newly developed AI can learn and understand the key features required for strong binding to a target protein, and design optimal drug candidate molecules—even without any prior input. This could significantly shift the paradigm of drug development.” He added, “Since this technology generates molecular structures based on principles of chemical interactions, it is expected to enable faster and more reliable drug development.”
Joongwon Lee and Wonho Zhung, PhD students in the Department of Chemistry, participated as co-first authors of this study. The research results were published in the international journal Advanced Science (IF = 14.1) on July 11.
● Paper Title: BInD: Bond and Interaction-Generating Diffusion Model for Multi-Objective Structure-Based Drug Design
● DOI: 10.1002/advs.202502702
This research was supported by the National Research Foundation of Korea and the Ministry of Health and Welfare.
2025.08.12 View 211
KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo >
Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model that, using only information about the target protein, can design optimal drug candidates without any prior molecular data—opening up new possibilities for drug discovery.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Professor Woo Youn Kim in the Department of Chemistry has developed an AI model named BInD (Bond and Interaction-generating Diffusion model), which can design and optimize drug candidate molecules tailored to a protein’s structure alone—without needing prior information about binding molecules. The model also predicts the binding mechanism (non-covalent interactions) between the drug and the target protein.
The core innovation of this technology lies in its “simultaneous design” approach. Previous AI models either focused on generating molecules or separately evaluating whether the generated molecule could bind to the target protein. In contrast, this new model considers the binding mechanism between the molecule and the protein during the generation process, enabling comprehensive design in one step. Since it pre-accounts for critical factors in protein-ligand binding, it has a much higher likelihood of generating effective and stable molecules. The generation process visually demonstrates how types and positions of atoms, covalent bonds, and interactions are created simultaneously to fit the protein’s binding site.
<Figure 1. Schematic of the diffusion model developed by the research team, which generates molecular structures and non-covalent interactions based on protein structures. Starting from a noise distribution, the model gradually removes noise (via reverse diffusion) to restore the atom positions, types, covalent bond types, and interaction types, thereby generating molecules. Interacting patterns are extracted from prior knowledge of known binding molecules or proteins, and through an inpainting technique, these patterns are kept fixed during the reverse diffusion process to guide the molecular generation.>
Moreover, this model is designed to meet multiple essential drug design criteria simultaneously—such as target binding affinity, drug-like properties, and structural stability. Traditional models often optimized for only one or two goals at the expense of others, but this new model balances various objectives, significantly enhancing its practical applicability.
The research team explained that the AI operates based on a “diffusion model”—a generative approach where a structure becomes increasingly refined from a random state. This is the same type of model used in AlphaFold 3, the 2024 Nobel Chemistry Prize-winning tool for protein-ligand structure generation, which has already demonstrated high efficiency.
Unlike AlphaFold 3, which provides spatial coordinates for atom positions, this study introduced a knowledge-based guide grounded in actual chemical laws—such as bond lengths and protein-ligand distances—enabling more chemically realistic structure generation.
<Figure 2. (Left) Target protein and the original bound molecule; (Right) Examples of molecules designed using the model developed in this study. The values for protein binding affinity (Vina), drug-likeness (QED), and synthetic accessibility (SA) are shown at the bottom.>
Additionally, the team applied an optimization strategy where outstanding binding patterns from prior results are reused. This allowed the model to generate even better drug candidates without additional training. Notably, the AI successfully produced molecules that selectively bind to the mutated residues of EGFR, a cancer-related target protein.
This study is also meaningful because it advances beyond the team’s previous research, which required prior input about the molecular conditions for the interaction pattern of protein binding.
Professor Woo Youn Kim commented that “the newly developed AI can learn and understand the key features required for strong binding to a target protein, and design optimal drug candidate molecules—even without any prior input. This could significantly shift the paradigm of drug development.” He added, “Since this technology generates molecular structures based on principles of chemical interactions, it is expected to enable faster and more reliable drug development.”
Joongwon Lee and Wonho Zhung, PhD students in the Department of Chemistry, participated as co-first authors of this study. The research results were published in the international journal Advanced Science (IF = 14.1) on July 11.
● Paper Title: BInD: Bond and Interaction-Generating Diffusion Model for Multi-Objective Structure-Based Drug Design
● DOI: 10.1002/advs.202502702
This research was supported by the National Research Foundation of Korea and the Ministry of Health and Welfare.
2025.08.12 View 211 -
 KAIST Develops Bioelectrosynthesis Platform for Switch-Like Precision Control of Cell Signaling
<(From left)Professor Jimin Park, Ph.D candidate Myeongeun Lee, Ph.D cadidate Jaewoong Lee,Professor Jihan Kim>
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally. A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
KAIST (President Kwang Hyung Lee) announced on August 11 that a research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim's group, has developed a 'Bioelectrosynthesis Platform' capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
Inspired by enzymes involved in nitrite reduction, the researchers implemented an electrochemical strategy that selectively produces nitric oxide or ammonia from a single precursor, nitrite (NO₂⁻). By changing the catalyst, the team generated ammonia or nitric oxide from nitrite using a copper-molybdenum-sulfur catalyst (Cu2MoS4) and an iron-incorporated catalyst (FeCuMS4), respectively.
Through electrochemical measurements and computer simulations, the team revealed that Fe sites in the FeCuMoS4 catalyst bind nitric oxide intermediates more strongly, shifting product selectivity toward nitric oxide. Under the same electrical conditions, the Fe-containing catalyst preferentially produces nitric oxide, whereas the Cu2MoS4 catalyst favors ammonia production.
<Figure 1. Schematic diagram of a bio-electrosynthesis platform that synthesizes a desired signaling substance with an electrical signal (left) and the results of precise cell control using it (right)>
The research team demonstrated biological functionality by using the platform to activate ion channels in human cells. Specifically, electrochemically produced nitric oxide activated TRPV1 channels (responsive to heat and chemical stimuli), while electrochemically produced ammonia induced intracellular alkalinization and activated OTOP1 proton channels. By tuning the applied voltage and electrolysis duration, the team modulated the onset time, spatial extent, and termination of cellular responses, which effectively turned cellular signaling on and off like a switch.
<Figure 2. Experimental results showing the change in the production ratio of nitric oxide and ammonia signaling substances according to the type of catalyst (left) and computational simulation results showing the strong bond between iron and nitric oxide (right)>
Professor Jimin Park said, "This work is significant because it enables precise cellular control by selectively producing signaling molecules with electricity. We believe it has strong potential for applications in electroceutical technologies targeting the nervous system or metabolic disorders."
Myeongeun Lee and Jaewoong Lee, Ph.D. students in the Department of Chemical and Biomolecular Engineering at KAIST, served as the co-first authors. Professor Jihan Kim is a co-author. The paper was published online in 'Angewandte Chemie International Edition' on July 8, 2025 (DOI: 10.1002/ange.202508192).
Reference: https://doi.org/10.1002/ange.202508192
Authors: Myeongeun Lee†, Jaewoong Lee†, Yongha Kim, Changho Lee, Sang Yeon Oh, Prof. Jihan Kim, Prof. Jimin Park*
†These authors contributed equally. *Corresponding author.
2025.08.12 View 124
KAIST Develops Bioelectrosynthesis Platform for Switch-Like Precision Control of Cell Signaling
<(From left)Professor Jimin Park, Ph.D candidate Myeongeun Lee, Ph.D cadidate Jaewoong Lee,Professor Jihan Kim>
Cells use various signaling molecules to regulate the nervous, immune, and vascular systems. Among these, nitric oxide (NO) and ammonia (NH₃) play important roles, but their chemical instability and gaseous nature make them difficult to generate or control externally. A KAIST research team has developed a platform that generates specific signaling molecules in situ from a single precursor under an applied electrical signal, enabling switch-like, precise spatiotemporal control of cellular responses. This approach could provide a foundation for future medical technologies such as electroceuticals, electrogenetics, and personalized cell therapies.
KAIST (President Kwang Hyung Lee) announced on August 11 that a research team led by Professor Jimin Park from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Jihan Kim's group, has developed a 'Bioelectrosynthesis Platform' capable of producing either nitric oxide or ammonia on demand using only an electrical signal. The platform allows control over the timing, spatial range, and duration of cell responses.
Inspired by enzymes involved in nitrite reduction, the researchers implemented an electrochemical strategy that selectively produces nitric oxide or ammonia from a single precursor, nitrite (NO₂⁻). By changing the catalyst, the team generated ammonia or nitric oxide from nitrite using a copper-molybdenum-sulfur catalyst (Cu2MoS4) and an iron-incorporated catalyst (FeCuMS4), respectively.
Through electrochemical measurements and computer simulations, the team revealed that Fe sites in the FeCuMoS4 catalyst bind nitric oxide intermediates more strongly, shifting product selectivity toward nitric oxide. Under the same electrical conditions, the Fe-containing catalyst preferentially produces nitric oxide, whereas the Cu2MoS4 catalyst favors ammonia production.
<Figure 1. Schematic diagram of a bio-electrosynthesis platform that synthesizes a desired signaling substance with an electrical signal (left) and the results of precise cell control using it (right)>
The research team demonstrated biological functionality by using the platform to activate ion channels in human cells. Specifically, electrochemically produced nitric oxide activated TRPV1 channels (responsive to heat and chemical stimuli), while electrochemically produced ammonia induced intracellular alkalinization and activated OTOP1 proton channels. By tuning the applied voltage and electrolysis duration, the team modulated the onset time, spatial extent, and termination of cellular responses, which effectively turned cellular signaling on and off like a switch.
<Figure 2. Experimental results showing the change in the production ratio of nitric oxide and ammonia signaling substances according to the type of catalyst (left) and computational simulation results showing the strong bond between iron and nitric oxide (right)>
Professor Jimin Park said, "This work is significant because it enables precise cellular control by selectively producing signaling molecules with electricity. We believe it has strong potential for applications in electroceutical technologies targeting the nervous system or metabolic disorders."
Myeongeun Lee and Jaewoong Lee, Ph.D. students in the Department of Chemical and Biomolecular Engineering at KAIST, served as the co-first authors. Professor Jihan Kim is a co-author. The paper was published online in 'Angewandte Chemie International Edition' on July 8, 2025 (DOI: 10.1002/ange.202508192).
Reference: https://doi.org/10.1002/ange.202508192
Authors: Myeongeun Lee†, Jaewoong Lee†, Yongha Kim, Changho Lee, Sang Yeon Oh, Prof. Jihan Kim, Prof. Jimin Park*
†These authors contributed equally. *Corresponding author.
2025.08.12 View 124 -
 2025 APEC Youth STEM Science Exchange Program Successfully Completed
<Photo1. Group photo at the end of the program>
KAIST (President Kwang Hyung Lee) announced on the 11thof August that it successfully hosted the 'APEC Youth STEM Conference KAIST Academic Program,' a global science exchange program for 28 youth researchers from 10 countries and over 30 experts who participated in the '2025 APEC Youth STEM* Collaborative Research and Competition.' The event was held at the main campus in Daejeon on Saturday, August 9.
STEM (Science, Technology, Engineering, Math) refers to the fields of science and engineering.
The competition was hosted by the Ministry of Science and ICT and organized by the APEC Science Gifted Mentoring Center. It took place from Wednesday, August 6, to Saturday, August 9, 2025, at KAIST in Daejeon and the Korea Science Academy of KAIST in Busan. The KAIST program was organized by the APEC Science Gifted Mentoring Center and supported by the KAIST Institute for the Gifted and Talented in Science Education.
Participants had the opportunity to experience Korea's cutting-edge research infrastructure firsthand, broaden their horizons in science and technology, and collaborate and exchange ideas with future science talents from the APEC region.
As the 2025 APEC chair, Korea is promoting various international collaborations to discover and nurture the next generation of talent in the STEM fields. The KAIST academic exchange program was particularly meaningful as it was designed with the international goal of revitalizing science gifted exchanges and expanding the basis for cooperation among APEC member countries. It moved beyond the traditional online-centric research collaboration model to focus on hands-on, on-site, and convergence research experiences.
The global science exchange program at KAIST introduced participants to KAIST's world-class educational and research environment and provided various academic content to allow them to experience real-world examples of convergence technology-based research.
<Photo2. Program Activities>
First, the KAIST Admissions Office participated, introducing KAIST's admissions system and its educational and research environment to outstanding international students, providing an opportunity to attract global talent. Following this, Dr. Tae-kyun Kwon of the Music and Audio Computing Lab at the Graduate School of Culture Technology presented a convergence art project based on musical artificial intelligence data, including a research demonstration in an anechoic chamber.
<Photo3. Participation in a music AI research demonstration>
Furthermore, a Climate Talk Concert program was organized under the leadership of the Graduate School of Green Growth and Sustainability, in connection with the theme of the APEC Youth STEM Collaborative Research: 'Youth-led STEM Solutions: Enhancing Climate Resilience.'
The program was planned and hosted by Dean Jiyong Eom. It provided a platform for young people to explore creative and practical STEM-based solutions to the climate crisis and seek opportunities for international cooperation.
<Photo4. Participation in Music AI Research Demonstration >
The program was a meaningful time for APEC youth researchers, offering practical support for their research through special lectures and Q&A sessions on:
Interdisciplinary Research and Education in the Era of Climate Crisis (Dean Jiyong Eom)
Energy Transition Technology in the Carbon Neutral Era (Professor Jeongrak Son)
Policies for Energy System Change (Professor Jihyo Kim)
Carbon Neutral Bio-technology (Professor Gyeongrok Choi)
After the afternoon talk concert, Lee Jing Jing, a student from Brunei, shared her thoughts, saying, "The lectures by the four professors were very meaningful and insightful. I was able to think about energy transition plans to solve climate change from various perspectives."
Si-jong Kwak, Director of the KAIST Global Institute for Talented Education, stated, "I hope that young people from all over the world will directly experience KAIST's research areas and environment, expand their interest in KAIST, and continue to grow as outstanding talents in the fields of science and engineering."
KAIST President Kwang Hyung Lee said, "KAIST will be at the center of science and technology-based international cooperation and will spare no effort to support future talents in developing creative and practical problem-solving skills. I hope this event served as an opportunity for young people to understand the value of global cooperation and grow into future science leaders."
2025.08.11 View 181
2025 APEC Youth STEM Science Exchange Program Successfully Completed
<Photo1. Group photo at the end of the program>
KAIST (President Kwang Hyung Lee) announced on the 11thof August that it successfully hosted the 'APEC Youth STEM Conference KAIST Academic Program,' a global science exchange program for 28 youth researchers from 10 countries and over 30 experts who participated in the '2025 APEC Youth STEM* Collaborative Research and Competition.' The event was held at the main campus in Daejeon on Saturday, August 9.
STEM (Science, Technology, Engineering, Math) refers to the fields of science and engineering.
The competition was hosted by the Ministry of Science and ICT and organized by the APEC Science Gifted Mentoring Center. It took place from Wednesday, August 6, to Saturday, August 9, 2025, at KAIST in Daejeon and the Korea Science Academy of KAIST in Busan. The KAIST program was organized by the APEC Science Gifted Mentoring Center and supported by the KAIST Institute for the Gifted and Talented in Science Education.
Participants had the opportunity to experience Korea's cutting-edge research infrastructure firsthand, broaden their horizons in science and technology, and collaborate and exchange ideas with future science talents from the APEC region.
As the 2025 APEC chair, Korea is promoting various international collaborations to discover and nurture the next generation of talent in the STEM fields. The KAIST academic exchange program was particularly meaningful as it was designed with the international goal of revitalizing science gifted exchanges and expanding the basis for cooperation among APEC member countries. It moved beyond the traditional online-centric research collaboration model to focus on hands-on, on-site, and convergence research experiences.
The global science exchange program at KAIST introduced participants to KAIST's world-class educational and research environment and provided various academic content to allow them to experience real-world examples of convergence technology-based research.
<Photo2. Program Activities>
First, the KAIST Admissions Office participated, introducing KAIST's admissions system and its educational and research environment to outstanding international students, providing an opportunity to attract global talent. Following this, Dr. Tae-kyun Kwon of the Music and Audio Computing Lab at the Graduate School of Culture Technology presented a convergence art project based on musical artificial intelligence data, including a research demonstration in an anechoic chamber.
<Photo3. Participation in a music AI research demonstration>
Furthermore, a Climate Talk Concert program was organized under the leadership of the Graduate School of Green Growth and Sustainability, in connection with the theme of the APEC Youth STEM Collaborative Research: 'Youth-led STEM Solutions: Enhancing Climate Resilience.'
The program was planned and hosted by Dean Jiyong Eom. It provided a platform for young people to explore creative and practical STEM-based solutions to the climate crisis and seek opportunities for international cooperation.
<Photo4. Participation in Music AI Research Demonstration >
The program was a meaningful time for APEC youth researchers, offering practical support for their research through special lectures and Q&A sessions on:
Interdisciplinary Research and Education in the Era of Climate Crisis (Dean Jiyong Eom)
Energy Transition Technology in the Carbon Neutral Era (Professor Jeongrak Son)
Policies for Energy System Change (Professor Jihyo Kim)
Carbon Neutral Bio-technology (Professor Gyeongrok Choi)
After the afternoon talk concert, Lee Jing Jing, a student from Brunei, shared her thoughts, saying, "The lectures by the four professors were very meaningful and insightful. I was able to think about energy transition plans to solve climate change from various perspectives."
Si-jong Kwak, Director of the KAIST Global Institute for Talented Education, stated, "I hope that young people from all over the world will directly experience KAIST's research areas and environment, expand their interest in KAIST, and continue to grow as outstanding talents in the fields of science and engineering."
KAIST President Kwang Hyung Lee said, "KAIST will be at the center of science and technology-based international cooperation and will spare no effort to support future talents in developing creative and practical problem-solving skills. I hope this event served as an opportunity for young people to understand the value of global cooperation and grow into future science leaders."
2025.08.11 View 181 -
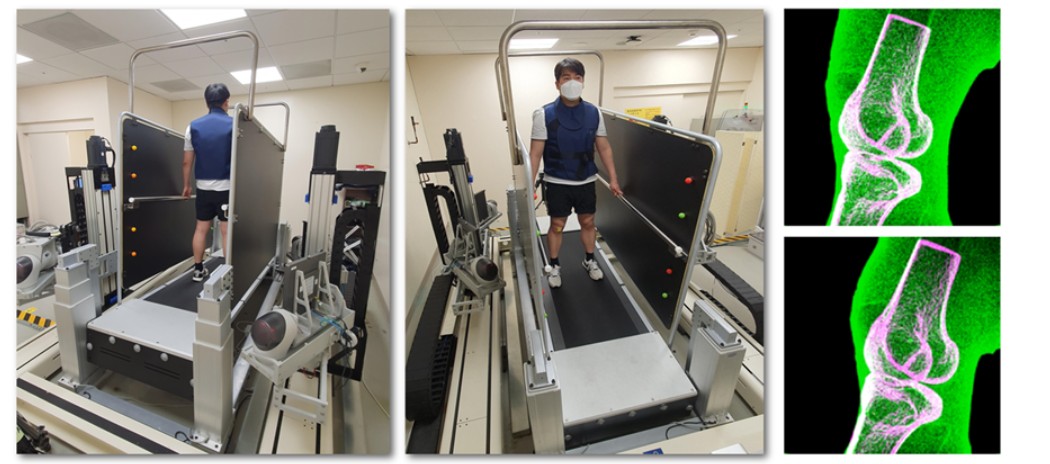 Prof. Seungbum Koo’s Team Receives Clinical Biomechanics Award at the 30th International Society of Biomechanics Conference
<(From Left) Ph.D candidate Jeongseok Oh from KAIST, Dr. Seungwoo Yoon from KAIST, Prof.Joon-Ho Wang from Samsung Medical Center, Prof.Seungbum Koo from KAIST>
Professor Seungbum Koo’s research team received the Clinical Biomechanics Award at the 30th International Society of Biomechanics (ISB) Conference, held in July 2025 in Stockholm, Sweden. The Plenary Lecture was delivered by first author and Ph.D. candidate Jeongseok Oh. This research was conducted in collaboration with Professor Joon-Ho Wang’s team at Samsung Medical Center.
Residual Translational and Rotational Kinematics After Combined ACL and Anterolateral Ligament Reconstruction During Walking
Jeongseok Oh, Seungwoo Yoon, Joon-Ho Wang, Seungbum Koo
The study analyzed gait-related knee joint motion using high-speed biplane X-ray imaging and three-dimensional kinematic reconstruction in 10 healthy individuals and 10 patients who underwent ACL reconstruction with ALL augmentation. The patient group showed excessive anterior translation and internal rotation, suggesting incomplete restoration of normal joint kinematics post-surgery. These findings provide mechanistic insight into the early onset of knee osteoarthritis often reported in this population.'
The ISB conference, held biennially for over 60 years, is the largest international biomechanics meeting. This year, it hosted 1,600 researchers from 46 countries and featured over 1,400 presentations. The Clinical Biomechanics Award is given to one outstanding study selected from five top-rated abstracts invited for full manuscript review. The winning paper is published in Clinical Biomechanics, and the award includes a monetary prize and a Plenary Lecture opportunity.
From 2019 to 2023, Koo and Wang’s teams developed a system with support from the Samsung Future Technology Development Program to track knee motion in real time during treadmill walking, using high-speed biplane X-rays and custom three-dimensional reconstruction software. This system, along with proprietary software that precisely reconstructs the three-dimensional motion of joints, was approved for clinical trials by the Ministry of Food and Drug Safety and installed at Samsung Medical Center. It is being used to quantitatively analyze abnormal joint motion patterns in patients with knee ligament injuries and those who have undergone knee surgery.
Additionally, Jeongseok Oh was named one of five finalists for the David Winter Young Investigator Award, presenting his work during the award session. This award recognizes promising young researchers in biomechanics worldwide.
2025.08.10 View 171
Prof. Seungbum Koo’s Team Receives Clinical Biomechanics Award at the 30th International Society of Biomechanics Conference
<(From Left) Ph.D candidate Jeongseok Oh from KAIST, Dr. Seungwoo Yoon from KAIST, Prof.Joon-Ho Wang from Samsung Medical Center, Prof.Seungbum Koo from KAIST>
Professor Seungbum Koo’s research team received the Clinical Biomechanics Award at the 30th International Society of Biomechanics (ISB) Conference, held in July 2025 in Stockholm, Sweden. The Plenary Lecture was delivered by first author and Ph.D. candidate Jeongseok Oh. This research was conducted in collaboration with Professor Joon-Ho Wang’s team at Samsung Medical Center.
Residual Translational and Rotational Kinematics After Combined ACL and Anterolateral Ligament Reconstruction During Walking
Jeongseok Oh, Seungwoo Yoon, Joon-Ho Wang, Seungbum Koo
The study analyzed gait-related knee joint motion using high-speed biplane X-ray imaging and three-dimensional kinematic reconstruction in 10 healthy individuals and 10 patients who underwent ACL reconstruction with ALL augmentation. The patient group showed excessive anterior translation and internal rotation, suggesting incomplete restoration of normal joint kinematics post-surgery. These findings provide mechanistic insight into the early onset of knee osteoarthritis often reported in this population.'
The ISB conference, held biennially for over 60 years, is the largest international biomechanics meeting. This year, it hosted 1,600 researchers from 46 countries and featured over 1,400 presentations. The Clinical Biomechanics Award is given to one outstanding study selected from five top-rated abstracts invited for full manuscript review. The winning paper is published in Clinical Biomechanics, and the award includes a monetary prize and a Plenary Lecture opportunity.
From 2019 to 2023, Koo and Wang’s teams developed a system with support from the Samsung Future Technology Development Program to track knee motion in real time during treadmill walking, using high-speed biplane X-rays and custom three-dimensional reconstruction software. This system, along with proprietary software that precisely reconstructs the three-dimensional motion of joints, was approved for clinical trials by the Ministry of Food and Drug Safety and installed at Samsung Medical Center. It is being used to quantitatively analyze abnormal joint motion patterns in patients with knee ligament injuries and those who have undergone knee surgery.
Additionally, Jeongseok Oh was named one of five finalists for the David Winter Young Investigator Award, presenting his work during the award session. This award recognizes promising young researchers in biomechanics worldwide.
2025.08.10 View 171 -
 KAIST’s Wearable Robot Design Wins ‘2025 Red Dot Award Best of the Best’
<Professor Hyunjoon Park, M.S candidate Eun-ju Kang, Prospective M.S candidate Jae-seong Kim, undergraduate student Min-su Kim>
A team led by Professor Hyunjoon Park from the Department of Industrial Design won the ‘Best of the Best’ award at the 2025 Red Dot Design Awards, one of the world's top three design awards, for their 'Angel Robotics WSF1 VISION Concept.'
The design for the next-generation wearable robot for people with paraplegia successfully implements functionality, aesthetics, and social inclusion. This latest achievement follows the team's iF Design Award win for the WalkON Suit F1 prototype, which also won a gold medal at the Cybathlon last year. This marks consecutive wins at top-tier international design awards.
KAIST (President Kwang-hyung Lee) announced on the 8th of August that Move Lab, a research team led by Professor Hyunjoon Park from the Department of Industrial Design, won the 'Best of the Best' award in the Design Concept-Professional category at the prestigious '2025 Red Dot Design Awards' for their next-generation wearable robot design, the ‘Angel Robotics WSF1 VISION Concept.’
The German 'Red Dot Design Awards' is one of the world's most well-known design competitions. It is considered one of the world's top three design awards along with Germany’s iF Design Awards and America’s IDEA. The ‘Best of the Best’ award is given to the best design in a category and is awarded only to a very select few of the top designs (within the top 1%) among all Red Dot Award winners.
Professor Hyunjoon Park’s team was honored with the ‘Best of the Best’ award for a user-friendly follow-up development of the ‘WalkON Suit F1 prototype,’ which won a gold medal at the 2024 Cybathlon and an iF Design Award in 2025.
<Figure 1. WSF1 Vision Concept Main Image>
This award-winning design is the result of industry-academic cooperation with Angel Robotics Inc., founded by Professor Kyoungchul Kong from the KAIST Department of Mechanical Engineering. It is a concept design that proposes a next-generation wearable robot (an ultra-personal mobility device) that can be used by people with paraplegia in their daily lives.
The research team focused on transforming Angel Robotics Inc.'s advanced engineering platform into an intuitive and emotional, user-centric experience, implementing a design solution that simultaneously possesses functionality, aesthetics, and social inclusion.
<Figure 2. WSF1 Vision Concept Full Exterior (Front View)>
The WSF1 VISION Concept includes innovative features implemented in Professor Kyoungchul Kong’s Exo Lab, such as:
An autonomous access function where the robot finds the user on its own.
A front-loading mechanism designed for the user to put it on alone while seated.
Multi-directional walking functionality realized through 12 powerful torque actuators and the latest control algorithms.
AI vision technology, along with a multi-visual display system that provides navigation and omnidirectional vision.
This provides users with a safer and more convenient mobility experience.
The strong yet elegant silhouette was achieved through a design process that pursued perfection in proportion, surfaces, and details not seen in existing wearable robots. In particular, the fabric cover that wraps around the entire thigh from the robot's hip joint is a stylish element that respects the wearer's self-esteem and individuality, like fashionable athletic wear. It also acts as a device for the wearer to psychologically feel safe in interacting with the robot and blending in with the general public. This presents a new aesthetic for wearable robots where function and form are harmonized.
<Figure 3. WSF1 Vision Concept's Operating Principle. It walks autonomously and is worn from the front while the user is seated.>
KAIST Professor Hyunjoon Park said of the award, "We are focusing on using technology, aesthetics, and human-centered innovation to present advanced technical solutions as easy, enjoyable, and cool experiences for users. Based on Angel Robotics Inc.'s vision of 'recreating human ability with technology,' the WSF1 VISION Concept aimed to break away from the traditional framework of wearable robots and deliver a design experience that adds dignity, independence, and new style to the user's life."
<Figure 4. WSF1 Vision Concept Detail Image>
A physical model of the WSF1 VISION Concept is scheduled to be unveiled in the Future Hall of the 2025 Gwangju Design Biennale from August 30 to November 2. The theme is 'Po-yong-ji-deok' (the virtue of inclusion), and it will showcase the role of design language in creating an inclusive future society.
<Figure 5. WSF1 Vision Concept: Image of a Person Wearing and Walking>
2025.08.09 View 121
KAIST’s Wearable Robot Design Wins ‘2025 Red Dot Award Best of the Best’
<Professor Hyunjoon Park, M.S candidate Eun-ju Kang, Prospective M.S candidate Jae-seong Kim, undergraduate student Min-su Kim>
A team led by Professor Hyunjoon Park from the Department of Industrial Design won the ‘Best of the Best’ award at the 2025 Red Dot Design Awards, one of the world's top three design awards, for their 'Angel Robotics WSF1 VISION Concept.'
The design for the next-generation wearable robot for people with paraplegia successfully implements functionality, aesthetics, and social inclusion. This latest achievement follows the team's iF Design Award win for the WalkON Suit F1 prototype, which also won a gold medal at the Cybathlon last year. This marks consecutive wins at top-tier international design awards.
KAIST (President Kwang-hyung Lee) announced on the 8th of August that Move Lab, a research team led by Professor Hyunjoon Park from the Department of Industrial Design, won the 'Best of the Best' award in the Design Concept-Professional category at the prestigious '2025 Red Dot Design Awards' for their next-generation wearable robot design, the ‘Angel Robotics WSF1 VISION Concept.’
The German 'Red Dot Design Awards' is one of the world's most well-known design competitions. It is considered one of the world's top three design awards along with Germany’s iF Design Awards and America’s IDEA. The ‘Best of the Best’ award is given to the best design in a category and is awarded only to a very select few of the top designs (within the top 1%) among all Red Dot Award winners.
Professor Hyunjoon Park’s team was honored with the ‘Best of the Best’ award for a user-friendly follow-up development of the ‘WalkON Suit F1 prototype,’ which won a gold medal at the 2024 Cybathlon and an iF Design Award in 2025.
<Figure 1. WSF1 Vision Concept Main Image>
This award-winning design is the result of industry-academic cooperation with Angel Robotics Inc., founded by Professor Kyoungchul Kong from the KAIST Department of Mechanical Engineering. It is a concept design that proposes a next-generation wearable robot (an ultra-personal mobility device) that can be used by people with paraplegia in their daily lives.
The research team focused on transforming Angel Robotics Inc.'s advanced engineering platform into an intuitive and emotional, user-centric experience, implementing a design solution that simultaneously possesses functionality, aesthetics, and social inclusion.
<Figure 2. WSF1 Vision Concept Full Exterior (Front View)>
The WSF1 VISION Concept includes innovative features implemented in Professor Kyoungchul Kong’s Exo Lab, such as:
An autonomous access function where the robot finds the user on its own.
A front-loading mechanism designed for the user to put it on alone while seated.
Multi-directional walking functionality realized through 12 powerful torque actuators and the latest control algorithms.
AI vision technology, along with a multi-visual display system that provides navigation and omnidirectional vision.
This provides users with a safer and more convenient mobility experience.
The strong yet elegant silhouette was achieved through a design process that pursued perfection in proportion, surfaces, and details not seen in existing wearable robots. In particular, the fabric cover that wraps around the entire thigh from the robot's hip joint is a stylish element that respects the wearer's self-esteem and individuality, like fashionable athletic wear. It also acts as a device for the wearer to psychologically feel safe in interacting with the robot and blending in with the general public. This presents a new aesthetic for wearable robots where function and form are harmonized.
<Figure 3. WSF1 Vision Concept's Operating Principle. It walks autonomously and is worn from the front while the user is seated.>
KAIST Professor Hyunjoon Park said of the award, "We are focusing on using technology, aesthetics, and human-centered innovation to present advanced technical solutions as easy, enjoyable, and cool experiences for users. Based on Angel Robotics Inc.'s vision of 'recreating human ability with technology,' the WSF1 VISION Concept aimed to break away from the traditional framework of wearable robots and deliver a design experience that adds dignity, independence, and new style to the user's life."
<Figure 4. WSF1 Vision Concept Detail Image>
A physical model of the WSF1 VISION Concept is scheduled to be unveiled in the Future Hall of the 2025 Gwangju Design Biennale from August 30 to November 2. The theme is 'Po-yong-ji-deok' (the virtue of inclusion), and it will showcase the role of design language in creating an inclusive future society.
<Figure 5. WSF1 Vision Concept: Image of a Person Wearing and Walking>
2025.08.09 View 121 -
 Key Figures in the Establishment of KAIST, Specially Invited to the Presidential Office’s National Appointment Ceremony
KAIST announced on August 6 that Professor Emeritus Jung-Woong Ra from the Department of Electrical Engineering and Won-ki Kwon, former Vice Minister of the Ministry of Science and Technology, who played pivotal roles in the establishment of KAIST, were selected as special guests for the 'National Appointment Ceremony' hosted by the Presidential Office on August 15th.
The Presidential Office selected special invitees across eight categories for the ceremony. These include individuals born in 1945 (referred to as 'Liberation Babies'), those involved in the founding of KAIST in 1971, independence activists and national patriots, overseas workers in Germany and the Middle East, AI industry professionals, residents from regions facing depopulation, leading figures in K-culture, military personnel, firefighters, police officers, families of fallen public servants and victims of social disasters, as well as promising talents in economics, science, culture, and the arts.
Considering the historical significance of its establishment and its symbolic meaning for the development of national science and technology, KAIST Professor Emeritus Jung-Woong Ra, who was a key figure in the establishment of the Department of Electrical Engineering after being appointed as a professor in 1971, and former Vice Minister Kwon Won-ki, who was the first practical leader of the establishment project. Both were officially included on the special invitation list.
Briefing from the Presidential Office regarding the 'National Appointment Ceremony' (2025.07.28) https://www.president.go.kr/newsroom/briefing/grehGMuP
2025.08.06 View 229
Key Figures in the Establishment of KAIST, Specially Invited to the Presidential Office’s National Appointment Ceremony
KAIST announced on August 6 that Professor Emeritus Jung-Woong Ra from the Department of Electrical Engineering and Won-ki Kwon, former Vice Minister of the Ministry of Science and Technology, who played pivotal roles in the establishment of KAIST, were selected as special guests for the 'National Appointment Ceremony' hosted by the Presidential Office on August 15th.
The Presidential Office selected special invitees across eight categories for the ceremony. These include individuals born in 1945 (referred to as 'Liberation Babies'), those involved in the founding of KAIST in 1971, independence activists and national patriots, overseas workers in Germany and the Middle East, AI industry professionals, residents from regions facing depopulation, leading figures in K-culture, military personnel, firefighters, police officers, families of fallen public servants and victims of social disasters, as well as promising talents in economics, science, culture, and the arts.
Considering the historical significance of its establishment and its symbolic meaning for the development of national science and technology, KAIST Professor Emeritus Jung-Woong Ra, who was a key figure in the establishment of the Department of Electrical Engineering after being appointed as a professor in 1971, and former Vice Minister Kwon Won-ki, who was the first practical leader of the establishment project. Both were officially included on the special invitation list.
Briefing from the Presidential Office regarding the 'National Appointment Ceremony' (2025.07.28) https://www.president.go.kr/newsroom/briefing/grehGMuP
2025.08.06 View 229 -
 Material Innovation Realized with Robotic Arms and AI, Without Human Researchers
<(From Left) M.S candidate Dongwoo Kim from KAIST, Ph.D candidate Hyun-Gi Lee from KAIST, Intern Yeham Kang from KAIST, M.S candidate Seongjae Bae from KAIST, Professor Dong-Hwa Seo from KAIST, (From top right, from left) Senior Researcher Inchul Park from POSCO Holdings, Senior Researcher Jung Woo Park, senior researcher from POSCO Holdings>
A joint research team from industry and academia in Korea has successfully developed an autonomous lab that uses AI and automation to create new cathode materials for secondary batteries. This system operates without human intervention, drastically reducing researcher labor and cutting the material discovery period by 93%.
* Autonomous Lab: A platform that autonomously designs, conducts, and analyzes experiments to find the optimal material.
KAIST (President Kwang Hyung Lee) announced on the 3rd of August that the research team led by Professor Dong-Hwa Seo of the Department of Materials Science and Engineering, in collaboration with the team of LIB Materials Research Center in Energy Materials R&D Laboratories at POSCO Holdings' POSCO N.EX.T Hub (Director Ki Soo Kim), built the lab to explore cathode materials using AI and automation technology.
Developing secondary battery cathode materials is a labor-intensive and time-consuming process for skilled researchers. It involves extensive exploration of various compositions and experimental variables through weighing, transporting, mixing, sintering*, and analyzing samples.
* Sintering: A process in which powder particles are heated to form a single solid mass through thermal activation.
The research team's autonomous lab combines an automated system with an AI model. The system handles all experimental steps—weighing, mixing, pelletizing, sintering, and analysis—without human interference. The AI model then interprets the data, learns from it, and selects the best candidates for the next experiment.
<Figure 1. Outline of the Anode Material Autonomous Exploration Laboratory>
To increase efficiency, the team designed the automation system with separate modules for each process, which are managed by a central robotic arm. This modular approach reduces the system's reliance on the robotic arm.
The team also significantly improved the synthesis speed by using a new high-speed sintering method, which is 50 times faster than the conventional low-speed method. This allows the autonomous lab to acquire 12 times more material data compared to traditional, researcher-led experiments.
<Figure 2. Synthesis of Cathode Material Using a High-Speed Sintering Device>
The vast amount of data collected is automatically interpreted by the AI model to extract information such as synthesized phases and impurity ratios. This data is systematically stored to create a high-quality database, which then serves as training data for an optimization AI model. This creates a closed-loop experimental system that recommends the next cathode composition and synthesis conditions for the automated system.
* Closed-loop experimental system: A system that independently performs all experimental processes without researcher intervention.
Operating this intelligent automation system 24 hours a day can secure more than 12 times the experimental data and shorten material discovery time by 93%. For a project requiring 500 experiments, the system can complete the work in about 6 days, whereas a traditional researcher-led approach would take 84 days.
During development, POSCO Holdings team managed the overall project planning, reviewed the platform design, and co-developed the partial module design and AI-based experimental model. The KAIST team, led by Professor Dong-hwa Seo, was responsible for the actual system implementation and operation, including platform design, module fabrication, algorithm creation, and system verification and improvement.
Professor Dong-Hwa Seo of KAIST stated that this system is a solution to the decrease in research personnel due to the low birth rate in Korea. He expects it will enhance global competitiveness by accelerating secondary battery material development through the acquisition of high-quality data.
<Figure 3. Exterior View (Side) of the Cathode Material Autonomous Exploration Laboratory>
POSCO N.EX.T Hub plans to apply an upgraded version of this autonomous lab to its own research facilities after 2026 to dramatically speed up next-generation secondary battery material development. They are planning further developments to enhance the system's stability and scalability, and hope this industry-academia collaboration will serve as a model for using innovative technology in real-world R&D.
<Figure 4. Exterior View (Front) of the Cathode Material Autonomous Exploration Laboratory>
The research was spearheaded by Ph.D. student Hyun-Gi Lee, along with master's students Seongjae Bae and Dongwoo Kim from Professor Dong-Hwa Seo’s lab at KAIST. Senior researchers Jung Woo Park and Inchul Park from LIB Materials Research Center of POSCO N.EX.T Hub's Energy Materials R&D Laboratories (Director Jeongjin Hong) also participated.
2025.08.06 View 313
Material Innovation Realized with Robotic Arms and AI, Without Human Researchers
<(From Left) M.S candidate Dongwoo Kim from KAIST, Ph.D candidate Hyun-Gi Lee from KAIST, Intern Yeham Kang from KAIST, M.S candidate Seongjae Bae from KAIST, Professor Dong-Hwa Seo from KAIST, (From top right, from left) Senior Researcher Inchul Park from POSCO Holdings, Senior Researcher Jung Woo Park, senior researcher from POSCO Holdings>
A joint research team from industry and academia in Korea has successfully developed an autonomous lab that uses AI and automation to create new cathode materials for secondary batteries. This system operates without human intervention, drastically reducing researcher labor and cutting the material discovery period by 93%.
* Autonomous Lab: A platform that autonomously designs, conducts, and analyzes experiments to find the optimal material.
KAIST (President Kwang Hyung Lee) announced on the 3rd of August that the research team led by Professor Dong-Hwa Seo of the Department of Materials Science and Engineering, in collaboration with the team of LIB Materials Research Center in Energy Materials R&D Laboratories at POSCO Holdings' POSCO N.EX.T Hub (Director Ki Soo Kim), built the lab to explore cathode materials using AI and automation technology.
Developing secondary battery cathode materials is a labor-intensive and time-consuming process for skilled researchers. It involves extensive exploration of various compositions and experimental variables through weighing, transporting, mixing, sintering*, and analyzing samples.
* Sintering: A process in which powder particles are heated to form a single solid mass through thermal activation.
The research team's autonomous lab combines an automated system with an AI model. The system handles all experimental steps—weighing, mixing, pelletizing, sintering, and analysis—without human interference. The AI model then interprets the data, learns from it, and selects the best candidates for the next experiment.
<Figure 1. Outline of the Anode Material Autonomous Exploration Laboratory>
To increase efficiency, the team designed the automation system with separate modules for each process, which are managed by a central robotic arm. This modular approach reduces the system's reliance on the robotic arm.
The team also significantly improved the synthesis speed by using a new high-speed sintering method, which is 50 times faster than the conventional low-speed method. This allows the autonomous lab to acquire 12 times more material data compared to traditional, researcher-led experiments.
<Figure 2. Synthesis of Cathode Material Using a High-Speed Sintering Device>
The vast amount of data collected is automatically interpreted by the AI model to extract information such as synthesized phases and impurity ratios. This data is systematically stored to create a high-quality database, which then serves as training data for an optimization AI model. This creates a closed-loop experimental system that recommends the next cathode composition and synthesis conditions for the automated system.
* Closed-loop experimental system: A system that independently performs all experimental processes without researcher intervention.
Operating this intelligent automation system 24 hours a day can secure more than 12 times the experimental data and shorten material discovery time by 93%. For a project requiring 500 experiments, the system can complete the work in about 6 days, whereas a traditional researcher-led approach would take 84 days.
During development, POSCO Holdings team managed the overall project planning, reviewed the platform design, and co-developed the partial module design and AI-based experimental model. The KAIST team, led by Professor Dong-hwa Seo, was responsible for the actual system implementation and operation, including platform design, module fabrication, algorithm creation, and system verification and improvement.
Professor Dong-Hwa Seo of KAIST stated that this system is a solution to the decrease in research personnel due to the low birth rate in Korea. He expects it will enhance global competitiveness by accelerating secondary battery material development through the acquisition of high-quality data.
<Figure 3. Exterior View (Side) of the Cathode Material Autonomous Exploration Laboratory>
POSCO N.EX.T Hub plans to apply an upgraded version of this autonomous lab to its own research facilities after 2026 to dramatically speed up next-generation secondary battery material development. They are planning further developments to enhance the system's stability and scalability, and hope this industry-academia collaboration will serve as a model for using innovative technology in real-world R&D.
<Figure 4. Exterior View (Front) of the Cathode Material Autonomous Exploration Laboratory>
The research was spearheaded by Ph.D. student Hyun-Gi Lee, along with master's students Seongjae Bae and Dongwoo Kim from Professor Dong-Hwa Seo’s lab at KAIST. Senior researchers Jung Woo Park and Inchul Park from LIB Materials Research Center of POSCO N.EX.T Hub's Energy Materials R&D Laboratories (Director Jeongjin Hong) also participated.
2025.08.06 View 313 -
 KAIST Develops AI ‘MARIOH’ to Uncover and Reconstruct Hidden Multi-Entity Relationships
<(From Left) Professor Kijung Shin, Ph.D candidate Kyuhan Lee, and Ph.D candidate Geon Lee>
Just like when multiple people gather simultaneously in a meeting room, higher-order interactions—where many entities interact at once—occur across various fields and reflect the complexity of real-world relationships. However, due to technical limitations, in many fields, only low-order pairwise interactions between entities can be observed and collected, which results in the loss of full context and restricts practical use. KAIST researchers have developed the AI model “MARIOH,” which can accurately reconstruct* higher-order interactions from such low-order information, opening up innovative analytical possibilities in fields like social network analysis, neuroscience, and life sciences.
*Reconstruction: Estimating/reconstructing the original structure that has disappeared or was not observed.
KAIST (President Kwang Hyung Lee) announced on the 5th that Professor Kijung Shin’s research team at the Kim Jaechul Graduate School of AI has developed an AI technology called “MARIOH” (Multiplicity-Aware Hypergraph Reconstruction), which can reconstruct higher-order interaction structures with high accuracy using only low-order interaction data.
Reconstructing higher-order interactions is challenging because a vast number of higher-order interactions can arise from the same low-order structure.
The key idea behind MARIOH, developed by the research team, is to utilize multiplicity information of low-order interactions to drastically reduce the number of candidate higher-order interactions that could stem from a given structure.
In addition, by employing efficient search techniques, MARIOH quickly identifies promising interaction candidates and uses multiplicity-based deep learning to accurately predict the likelihood that each candidate represents an actual higher-order interaction.
<Figure 1. An example of recovering high-dimensional relationships (right) from low-dimensional paper co-authorship relationships (left) with 100% accuracy, using MARIOH technology.>
Through experiments on ten diverse real-world datasets, the research team showed that MARIOH reconstructed higher-order interactions with up to 74% greater accuracy compared to existing methods.
For instance, in a dataset on co-authorship relations (source: DBLP), MARIOH achieved a reconstruction accuracy of over 98%, significantly outperforming existing methods, which reached only about 86%. Furthermore, leveraging the reconstructed higher-order structures led to improved performance in downstream tasks, including prediction and classification.
According to Kijung, “MARIOH moves beyond existing approaches that rely solely on simplified connection information, enabling precise analysis of the complex interconnections found in the real world.” Furthermore, “it has broad potential applications in fields such as social network analysis for group chats or collaborative networks, life sciences for studying protein complexes or gene interactions, and neuroscience for tracking simultaneous activity across multiple brain regions.”
The research was conducted by Kyuhan Lee (Integrated M.S.–Ph.D. program at the Kim Jaechul Graduate School of AI at KAIST; currently a software engineer at GraphAI), Geon Lee (Integrated M.S.–Ph.D. program at KAIST), and Professor Kijung Shin. It was presented at the 41st IEEE International Conference on Data Engineering (IEEE ICDE), held in Hong Kong this past May.
※ Paper title: MARIOH: Multiplicity-Aware Hypergraph Reconstruction ※ DOI: https://doi.ieeecomputersociety.org/10.1109/ICDE65448.2025.00233
<Figure 2. An example of the process of recovering high-dimensional relationships using MARIOH technology>
This research was supported by the Institute of Information & Communications Technology Planning & Evaluation (IITP) through the project “EntireDB2AI: Foundational technologies and software for deep representation learning and prediction using complete relational databases,” as well as by the National Research Foundation of Korea through the project “Graph Foundation Model: Graph-based machine learning applicable across various modalities and domains.”
2025.08.05 View 331
KAIST Develops AI ‘MARIOH’ to Uncover and Reconstruct Hidden Multi-Entity Relationships
<(From Left) Professor Kijung Shin, Ph.D candidate Kyuhan Lee, and Ph.D candidate Geon Lee>
Just like when multiple people gather simultaneously in a meeting room, higher-order interactions—where many entities interact at once—occur across various fields and reflect the complexity of real-world relationships. However, due to technical limitations, in many fields, only low-order pairwise interactions between entities can be observed and collected, which results in the loss of full context and restricts practical use. KAIST researchers have developed the AI model “MARIOH,” which can accurately reconstruct* higher-order interactions from such low-order information, opening up innovative analytical possibilities in fields like social network analysis, neuroscience, and life sciences.
*Reconstruction: Estimating/reconstructing the original structure that has disappeared or was not observed.
KAIST (President Kwang Hyung Lee) announced on the 5th that Professor Kijung Shin’s research team at the Kim Jaechul Graduate School of AI has developed an AI technology called “MARIOH” (Multiplicity-Aware Hypergraph Reconstruction), which can reconstruct higher-order interaction structures with high accuracy using only low-order interaction data.
Reconstructing higher-order interactions is challenging because a vast number of higher-order interactions can arise from the same low-order structure.
The key idea behind MARIOH, developed by the research team, is to utilize multiplicity information of low-order interactions to drastically reduce the number of candidate higher-order interactions that could stem from a given structure.
In addition, by employing efficient search techniques, MARIOH quickly identifies promising interaction candidates and uses multiplicity-based deep learning to accurately predict the likelihood that each candidate represents an actual higher-order interaction.
<Figure 1. An example of recovering high-dimensional relationships (right) from low-dimensional paper co-authorship relationships (left) with 100% accuracy, using MARIOH technology.>
Through experiments on ten diverse real-world datasets, the research team showed that MARIOH reconstructed higher-order interactions with up to 74% greater accuracy compared to existing methods.
For instance, in a dataset on co-authorship relations (source: DBLP), MARIOH achieved a reconstruction accuracy of over 98%, significantly outperforming existing methods, which reached only about 86%. Furthermore, leveraging the reconstructed higher-order structures led to improved performance in downstream tasks, including prediction and classification.
According to Kijung, “MARIOH moves beyond existing approaches that rely solely on simplified connection information, enabling precise analysis of the complex interconnections found in the real world.” Furthermore, “it has broad potential applications in fields such as social network analysis for group chats or collaborative networks, life sciences for studying protein complexes or gene interactions, and neuroscience for tracking simultaneous activity across multiple brain regions.”
The research was conducted by Kyuhan Lee (Integrated M.S.–Ph.D. program at the Kim Jaechul Graduate School of AI at KAIST; currently a software engineer at GraphAI), Geon Lee (Integrated M.S.–Ph.D. program at KAIST), and Professor Kijung Shin. It was presented at the 41st IEEE International Conference on Data Engineering (IEEE ICDE), held in Hong Kong this past May.
※ Paper title: MARIOH: Multiplicity-Aware Hypergraph Reconstruction ※ DOI: https://doi.ieeecomputersociety.org/10.1109/ICDE65448.2025.00233
<Figure 2. An example of the process of recovering high-dimensional relationships using MARIOH technology>
This research was supported by the Institute of Information & Communications Technology Planning & Evaluation (IITP) through the project “EntireDB2AI: Foundational technologies and software for deep representation learning and prediction using complete relational databases,” as well as by the National Research Foundation of Korea through the project “Graph Foundation Model: Graph-based machine learning applicable across various modalities and domains.”
2025.08.05 View 331 -
 Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim>
"Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
*Chemical Synthesis: A process of creating desired substances using chemical reactions.
KAIST (President Kwang Hyung Lee) announced on the 31st of July that a research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances 'herpotrichones A, B, and C' for the first time.
Herpotrichone natural products are substances obtainable only in minute quantities from 'Herpotrichia sp. SF09', a symbiotic pill bug fungus, and possess a unique 6/6/6/6/3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).
Interestingly, this substance exhibits excellent anti-neuroinflammatory effects that suppress brain inflammatory reactions. Recently, its mechanism of action to protect nerve cells by inhibiting ferroptosis (iron-mediated cell death) was also reported, raising expectations for its potential as a therapeutic drug for brain diseases.
Professor Han's research team devised a biosynthetically inspired strategy to chemically synthesize herpotrichoneS. The key to success was a named chemical reaction "Diels-Alder (DA) reaction". This reaction forms a six-membered ring by creating new bonds between carbon-based partners, much like two puzzle pieces interlocking to form a single ring.
<Figure 2. Key Synthetic Strategy for Hypotricon A, B, and C Based on Hydrogen Bonding>
Furthermore, the research team focused on a weak attractive phenomenon between molecules called "hydrogen bonding". By delicately designing and controlling this hydrogen bond, they were able to precisely induce the reaction to occur chemo-, regio- and stereoselectively, thereby synthesizing herpotrichone. Notably, without the pivotal hydrogen bond, only a small amount of the target natural product was formed or only undesirable byproducts were generated.
The configuration of the C2’ hydroxyl moiety was essential in directing the desired transition states leading to the target natural products.
Thanks to this induced hydrogen bonding, the reacting molecules approached the correct positions and went through an ideal transition state, allowing for the synthesis of herpotrichone C. This reaction principle was also successfully applied to herpotrichone A and B, enabling the successful synthesis of these natural products.
During the key Diels-Alder reaction conducted in the laboratory, new molecular structures not yet discovered in nature were also formed. Some of these have a high probability of being novel natural products with excellent pharmacological activity, thus doubling the significance of this research for anticipating natural products through synthesis.
Indeed, while Professor Han's research team conducted synthetic studies on herpotrichone A and B based on a 2019 paper by Chinese researchers who discovered and elucidated their structures, the research team observed the formation of undesired byproducts.
Interestingly, in 2024, the same Chinese research team that discovered herpotrichones A and bn reported the discovery of a new natural product called herpotrichone C, which turned out to be the same substance as the major byproduct previously obtained by Professor Han's team en route to herpotrichones A and B.
Professor Han stated, "This is the first total synthesis of a rare natural product with pharmacological activity related to neurodegenerative diseases and systematically presents the principle of biomimetic synthesis of complex natural products." He added, "It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products."
This research outcome, with Yoojin Lee, a master's and Ph.D. integrated course student in the Department of Chemistry, as the first author, was published on July 16th in the Journal of the American Chemical Society (JACS), one of the most prestigious academic journals in the field of chemistry.
This research was supported by the National Research Foundation of Korea (NRF) Mid-career Researcher Support Program, the KAIST UP Project, the KAIST Grand Challenge 30 Project, and the KAIST Trans-Generational Collaborative Research Laboratory Project.
2025.08.04 View 419
Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim>
"Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
*Chemical Synthesis: A process of creating desired substances using chemical reactions.
KAIST (President Kwang Hyung Lee) announced on the 31st of July that a research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances 'herpotrichones A, B, and C' for the first time.
Herpotrichone natural products are substances obtainable only in minute quantities from 'Herpotrichia sp. SF09', a symbiotic pill bug fungus, and possess a unique 6/6/6/6/3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).
Interestingly, this substance exhibits excellent anti-neuroinflammatory effects that suppress brain inflammatory reactions. Recently, its mechanism of action to protect nerve cells by inhibiting ferroptosis (iron-mediated cell death) was also reported, raising expectations for its potential as a therapeutic drug for brain diseases.
Professor Han's research team devised a biosynthetically inspired strategy to chemically synthesize herpotrichoneS. The key to success was a named chemical reaction "Diels-Alder (DA) reaction". This reaction forms a six-membered ring by creating new bonds between carbon-based partners, much like two puzzle pieces interlocking to form a single ring.
<Figure 2. Key Synthetic Strategy for Hypotricon A, B, and C Based on Hydrogen Bonding>
Furthermore, the research team focused on a weak attractive phenomenon between molecules called "hydrogen bonding". By delicately designing and controlling this hydrogen bond, they were able to precisely induce the reaction to occur chemo-, regio- and stereoselectively, thereby synthesizing herpotrichone. Notably, without the pivotal hydrogen bond, only a small amount of the target natural product was formed or only undesirable byproducts were generated.
The configuration of the C2’ hydroxyl moiety was essential in directing the desired transition states leading to the target natural products.
Thanks to this induced hydrogen bonding, the reacting molecules approached the correct positions and went through an ideal transition state, allowing for the synthesis of herpotrichone C. This reaction principle was also successfully applied to herpotrichone A and B, enabling the successful synthesis of these natural products.
During the key Diels-Alder reaction conducted in the laboratory, new molecular structures not yet discovered in nature were also formed. Some of these have a high probability of being novel natural products with excellent pharmacological activity, thus doubling the significance of this research for anticipating natural products through synthesis.
Indeed, while Professor Han's research team conducted synthetic studies on herpotrichone A and B based on a 2019 paper by Chinese researchers who discovered and elucidated their structures, the research team observed the formation of undesired byproducts.
Interestingly, in 2024, the same Chinese research team that discovered herpotrichones A and bn reported the discovery of a new natural product called herpotrichone C, which turned out to be the same substance as the major byproduct previously obtained by Professor Han's team en route to herpotrichones A and B.
Professor Han stated, "This is the first total synthesis of a rare natural product with pharmacological activity related to neurodegenerative diseases and systematically presents the principle of biomimetic synthesis of complex natural products." He added, "It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products."
This research outcome, with Yoojin Lee, a master's and Ph.D. integrated course student in the Department of Chemistry, as the first author, was published on July 16th in the Journal of the American Chemical Society (JACS), one of the most prestigious academic journals in the field of chemistry.
This research was supported by the National Research Foundation of Korea (NRF) Mid-career Researcher Support Program, the KAIST UP Project, the KAIST Grand Challenge 30 Project, and the KAIST Trans-Generational Collaborative Research Laboratory Project.
2025.08.04 View 419 -
 Is 24-hour health monitoring possible with ambient light energy?
<(From left) Ph.D candidate Youngmin Sim, Ph.D candidate Do Yun Park, Dr. Chanho Park, Professor Kyeongha Kwon>
Miniaturization and weight reduction of medical wearable devices for continuous health monitoring such as heart rate, blood oxygen saturation, and sweat component analysis remain major challenges. In particular, optical sensors consume a significant amount of power for LED operation and wireless transmission, requiring heavy and bulky batteries. To overcome these limitations, KAIST researchers have developed a next-generation wearable platform that enables 24-hour continuous measurement by using ambient light as an energy source and optimizing power management according to the power environment.
KAIST (President Kwang Hyung Lee) announced on the 30th that Professor Kyeongha Kwon's team from the School of Electrical Engineering, in collaboration with Dr. Chanho Park’s team at Northwestern University in the U.S., has developed an adaptive wireless wearable platform that reduces battery load by utilizing ambient light.
To address the battery issue of medical wearable devices, Professor Kyeongha Kwon’s research team developed an innovative platform that utilizes ambient natural light as an energy source. This platform integrates three complementary light energy technologies.
<Figure1.The wireless wearable platform minimizes the energy required for light sources through i) Photometric system that directly utilizes ambient light passing through windows for measurements, ii) Photovoltaic system that receives power from high-efficiency photovoltaic cells and wireless power receiver coils, and iii) Photoluminescent system that stores light using photoluminescent materials and emits light in dark conditions to support the two aforementioned systems. In-sensor computing minimizes power consumption by wirelessly transmitting only essential data. The adaptive power management system efficiently manages power by automatically selecting the optimal mode among 11 different power modes through a power selector based on the power supply level from the photovoltaic system and battery charge status.>
The first core technology, the Photometric Method, is a technique that adaptively adjusts LED brightness depending on the intensity of the ambient light source. By combining ambient natural light with LED light to maintain a constant total illumination level, it automatically dims the LED when natural light is strong and brightens it when natural light is weak.
Whereas conventional sensors had to keep the LED on at a fixed brightness regardless of the environment, this technology optimizes LED power in real time according to the surrounding environment. Experimental results showed that it reduced power consumption by as much as 86.22% under sufficient lighting conditions.
The second is the Photovoltaic Method using high-efficiency multijunction solar cells. This goes beyond simple solar power generation to convert light in both indoor and outdoor environments into electricity. In particular, the adaptive power management system automatically switches among 11 different power configurations based on ambient conditions and battery status to achieve optimal energy efficiency.
The third innovative technology is the Photoluminescent Method. By mixing strontium aluminate microparticles* into the sensor’s silicone encapsulation structure, light from the surroundings is absorbed and stored during the day and slowly released in the dark. As a result, after being exposed to 500W/m² of sunlight for 10 minutes, continuous measurement is possible for 2.5 minutes even in complete darkness.
*Strontium aluminate microparticles: A photoluminescent material used in glow-in-the-dark paint or safety signs, which absorbs light and emits it in the dark for an extended time.
These three technologies work complementarily—during bright conditions, the first and second methods are active, and in dark conditions, the third method provides additional support—enabling 24-hour continuous operation.
The research team applied this platform to various medical sensors to verify its practicality. The photoplethysmography sensor monitors heart rate and blood oxygen saturation in real time, allowing early detection of cardiovascular diseases. The blue light dosimeter accurately measures blue light, which causes skin aging and damage, and provides personalized skin protection guidance. The sweat analysis sensor uses microfluidic technology to simultaneously analyze salt, glucose, and pH in sweat, enabling real-time detection of dehydration and electrolyte imbalances.
Additionally, introducing in-sensor data computing significantly reduced wireless communication power consumption. Previously, all raw data had to be transmitted externally, but now only the necessary results are calculated and transmitted within the sensor, reducing data transmission requirements from 400B/s to 4B/s—a 100-fold decrease.
To validate performance, the research tested the device on healthy adult subjects in four different environments: bright indoor lighting, dim lighting, infrared lighting, and complete darkness. The results showed measurement accuracy equivalent to that of commercial medical devices in all conditions A mouse model experiment confirmed accurate blood oxygen saturation measurement in hypoxic conditions.
<Frigure2.The multimodal device applying the energy harvesting and power management platform consists of i) photoplethysmography (PPG) sensor, ii) blue light dosimeter, iii) photoluminescent microfluidic channel for sweat analysis and biomarker sensors (chloride ion, glucose, and pH), and iv) temperature sensor. This device was implemented with flexible printed circuit board (fPCB) to enable attachment to the skin. A silicon substrate with a window that allows ambient light and measurement light to pass through, along with photoluminescent encapsulation layer, encapsulates the PPG, blue light dosimeter, and temperature sensors, while the photoluminescent microfluidic channel is attached below the photoluminescent encapsulation layer to collect sweat>
Professor Kyeongha Kwon of KAIST, who led the research, stated, “This technology will enable 24-hour continuous health monitoring, shifting the medical paradigm from treatment-centered to prevention-centered shifting the medical paradigm from treatment-centered to prevention-centered,” further stating that “cost savings through early diagnosis as well as strengthened technological competitiveness in the next-generation wearable healthcare market are anticipated.”
This research was published on July 1 in the international journal Nature Communications, with Do Yun Park, a doctoral student in the AI Semiconductor Graduate Program, as co–first author.
※ Paper title: Adaptive Electronics for Photovoltaic, Photoluminescent and Photometric Methods in Power Harvesting for Wireless and Wearable Sensors ※ DOI: https://doi.org/10.1038/s41467-025-60911-1 ※ URL: https://www.nature.com/articles/s41467-025-60911-1
This research was supported by the National Research Foundation of Korea (Outstanding Young Researcher Program and Regional Innovation Leading Research Center Project), the Ministry of Science and ICT and Institute of Information & Communications Technology Planning & Evaluation (IITP) AI Semiconductor Graduate Program, and the BK FOUR Program (Connected AI Education & Research Program for Industry and Society Innovation, KAIST EE).
2025.07.30 View 565
Is 24-hour health monitoring possible with ambient light energy?
<(From left) Ph.D candidate Youngmin Sim, Ph.D candidate Do Yun Park, Dr. Chanho Park, Professor Kyeongha Kwon>
Miniaturization and weight reduction of medical wearable devices for continuous health monitoring such as heart rate, blood oxygen saturation, and sweat component analysis remain major challenges. In particular, optical sensors consume a significant amount of power for LED operation and wireless transmission, requiring heavy and bulky batteries. To overcome these limitations, KAIST researchers have developed a next-generation wearable platform that enables 24-hour continuous measurement by using ambient light as an energy source and optimizing power management according to the power environment.
KAIST (President Kwang Hyung Lee) announced on the 30th that Professor Kyeongha Kwon's team from the School of Electrical Engineering, in collaboration with Dr. Chanho Park’s team at Northwestern University in the U.S., has developed an adaptive wireless wearable platform that reduces battery load by utilizing ambient light.
To address the battery issue of medical wearable devices, Professor Kyeongha Kwon’s research team developed an innovative platform that utilizes ambient natural light as an energy source. This platform integrates three complementary light energy technologies.
<Figure1.The wireless wearable platform minimizes the energy required for light sources through i) Photometric system that directly utilizes ambient light passing through windows for measurements, ii) Photovoltaic system that receives power from high-efficiency photovoltaic cells and wireless power receiver coils, and iii) Photoluminescent system that stores light using photoluminescent materials and emits light in dark conditions to support the two aforementioned systems. In-sensor computing minimizes power consumption by wirelessly transmitting only essential data. The adaptive power management system efficiently manages power by automatically selecting the optimal mode among 11 different power modes through a power selector based on the power supply level from the photovoltaic system and battery charge status.>
The first core technology, the Photometric Method, is a technique that adaptively adjusts LED brightness depending on the intensity of the ambient light source. By combining ambient natural light with LED light to maintain a constant total illumination level, it automatically dims the LED when natural light is strong and brightens it when natural light is weak.
Whereas conventional sensors had to keep the LED on at a fixed brightness regardless of the environment, this technology optimizes LED power in real time according to the surrounding environment. Experimental results showed that it reduced power consumption by as much as 86.22% under sufficient lighting conditions.
The second is the Photovoltaic Method using high-efficiency multijunction solar cells. This goes beyond simple solar power generation to convert light in both indoor and outdoor environments into electricity. In particular, the adaptive power management system automatically switches among 11 different power configurations based on ambient conditions and battery status to achieve optimal energy efficiency.
The third innovative technology is the Photoluminescent Method. By mixing strontium aluminate microparticles* into the sensor’s silicone encapsulation structure, light from the surroundings is absorbed and stored during the day and slowly released in the dark. As a result, after being exposed to 500W/m² of sunlight for 10 minutes, continuous measurement is possible for 2.5 minutes even in complete darkness.
*Strontium aluminate microparticles: A photoluminescent material used in glow-in-the-dark paint or safety signs, which absorbs light and emits it in the dark for an extended time.
These three technologies work complementarily—during bright conditions, the first and second methods are active, and in dark conditions, the third method provides additional support—enabling 24-hour continuous operation.
The research team applied this platform to various medical sensors to verify its practicality. The photoplethysmography sensor monitors heart rate and blood oxygen saturation in real time, allowing early detection of cardiovascular diseases. The blue light dosimeter accurately measures blue light, which causes skin aging and damage, and provides personalized skin protection guidance. The sweat analysis sensor uses microfluidic technology to simultaneously analyze salt, glucose, and pH in sweat, enabling real-time detection of dehydration and electrolyte imbalances.
Additionally, introducing in-sensor data computing significantly reduced wireless communication power consumption. Previously, all raw data had to be transmitted externally, but now only the necessary results are calculated and transmitted within the sensor, reducing data transmission requirements from 400B/s to 4B/s—a 100-fold decrease.
To validate performance, the research tested the device on healthy adult subjects in four different environments: bright indoor lighting, dim lighting, infrared lighting, and complete darkness. The results showed measurement accuracy equivalent to that of commercial medical devices in all conditions A mouse model experiment confirmed accurate blood oxygen saturation measurement in hypoxic conditions.
<Frigure2.The multimodal device applying the energy harvesting and power management platform consists of i) photoplethysmography (PPG) sensor, ii) blue light dosimeter, iii) photoluminescent microfluidic channel for sweat analysis and biomarker sensors (chloride ion, glucose, and pH), and iv) temperature sensor. This device was implemented with flexible printed circuit board (fPCB) to enable attachment to the skin. A silicon substrate with a window that allows ambient light and measurement light to pass through, along with photoluminescent encapsulation layer, encapsulates the PPG, blue light dosimeter, and temperature sensors, while the photoluminescent microfluidic channel is attached below the photoluminescent encapsulation layer to collect sweat>
Professor Kyeongha Kwon of KAIST, who led the research, stated, “This technology will enable 24-hour continuous health monitoring, shifting the medical paradigm from treatment-centered to prevention-centered shifting the medical paradigm from treatment-centered to prevention-centered,” further stating that “cost savings through early diagnosis as well as strengthened technological competitiveness in the next-generation wearable healthcare market are anticipated.”
This research was published on July 1 in the international journal Nature Communications, with Do Yun Park, a doctoral student in the AI Semiconductor Graduate Program, as co–first author.
※ Paper title: Adaptive Electronics for Photovoltaic, Photoluminescent and Photometric Methods in Power Harvesting for Wireless and Wearable Sensors ※ DOI: https://doi.org/10.1038/s41467-025-60911-1 ※ URL: https://www.nature.com/articles/s41467-025-60911-1
This research was supported by the National Research Foundation of Korea (Outstanding Young Researcher Program and Regional Innovation Leading Research Center Project), the Ministry of Science and ICT and Institute of Information & Communications Technology Planning & Evaluation (IITP) AI Semiconductor Graduate Program, and the BK FOUR Program (Connected AI Education & Research Program for Industry and Society Innovation, KAIST EE).
2025.07.30 View 565 -
 KAIST GESS Team Awarded Honorable Mention at 2025 Entrepreneurship Olympiad
<Photo: eaureco team at the final pitch>
The KAIST Global Entrepreneurship Summer School (GESS) winning team, eaureco, earned an Honorable Mention at the 2025 Entrepreneurship Olympiad, held July 21–23 at Stanford Faculty Club and hosted by Techdev Academy. Competing in the college track, the team showcased their innovative solution among participants from top institutions including Stanford University, UC Berkeley, UCLA, and UC San Diego.
Team eaureco—comprising KAIST undergraduate and graduate students Jiwon Park(Semiconductor Systems Engineering), Si Li Sara (Julia) Aow, Lunar Sebastian Widjaja (both Civil & Environmental Engineering), Seoyeon Jang (Impact MBA), and Isabel Alexandra Cornejo Lima (BTM/Global Digital Innovation)—presented a B2B solution that upcycles discarded seaweed into biodegradable ice packs for cold-chain companies. Their business model was recognized for its alignment with sustainability, resource circulation, and social impact goals.
<Photo: eaureco team preparing for the final pitch>
The team’s ability to rapidly adapt their pitch based on mentor feedback and clearly communicate the value of their idea to judges contributed to their recognition. This accomplishment further highlights the impact of KAIST's GESS program, which supports students in building real-world entrepreneurial skills through immersive learning experiences in Silicon Valley.
“The GESS program helped us refine every aspect of our business idea—from identifying the problem to developing a go-to-market strategy,” said Si Li Sara (Julia) Aow, a member of the eaureco team. “We’re grateful for the opportunity to showcase our work on a global stage and hope to continue developing innovations that drive meaningful change.”
“This award reaffirms the creative potential and practical capabilities of KAIST students in global innovation ecosystems,” said Dr. Soyoung Kim, Vice President of International Office. “We will continue to invest in programs like GESS to empower our students as future leaders in entrepreneurship.”
The Entrepreneurship Olympiad is a global event designed to foster innovation, entrepreneurship, and collaboration among young change-makers. This year’s program featured keynote talks, panels, and workshops led by industry pioneers including Marc Tarpenning (Co-founder, Tesla Motors), Pat Brown (Founder, Impossible Foods), and other influential entrepreneurs from the biotech, fintech, and deeptech sectors.
The Honorable Mention recognition underscores KAIST’s commitment to global entrepreneurship education and the growing international visibility of the GESS program.
2025.07.29 View 499
KAIST GESS Team Awarded Honorable Mention at 2025 Entrepreneurship Olympiad
<Photo: eaureco team at the final pitch>
The KAIST Global Entrepreneurship Summer School (GESS) winning team, eaureco, earned an Honorable Mention at the 2025 Entrepreneurship Olympiad, held July 21–23 at Stanford Faculty Club and hosted by Techdev Academy. Competing in the college track, the team showcased their innovative solution among participants from top institutions including Stanford University, UC Berkeley, UCLA, and UC San Diego.
Team eaureco—comprising KAIST undergraduate and graduate students Jiwon Park(Semiconductor Systems Engineering), Si Li Sara (Julia) Aow, Lunar Sebastian Widjaja (both Civil & Environmental Engineering), Seoyeon Jang (Impact MBA), and Isabel Alexandra Cornejo Lima (BTM/Global Digital Innovation)—presented a B2B solution that upcycles discarded seaweed into biodegradable ice packs for cold-chain companies. Their business model was recognized for its alignment with sustainability, resource circulation, and social impact goals.
<Photo: eaureco team preparing for the final pitch>
The team’s ability to rapidly adapt their pitch based on mentor feedback and clearly communicate the value of their idea to judges contributed to their recognition. This accomplishment further highlights the impact of KAIST's GESS program, which supports students in building real-world entrepreneurial skills through immersive learning experiences in Silicon Valley.
“The GESS program helped us refine every aspect of our business idea—from identifying the problem to developing a go-to-market strategy,” said Si Li Sara (Julia) Aow, a member of the eaureco team. “We’re grateful for the opportunity to showcase our work on a global stage and hope to continue developing innovations that drive meaningful change.”
“This award reaffirms the creative potential and practical capabilities of KAIST students in global innovation ecosystems,” said Dr. Soyoung Kim, Vice President of International Office. “We will continue to invest in programs like GESS to empower our students as future leaders in entrepreneurship.”
The Entrepreneurship Olympiad is a global event designed to foster innovation, entrepreneurship, and collaboration among young change-makers. This year’s program featured keynote talks, panels, and workshops led by industry pioneers including Marc Tarpenning (Co-founder, Tesla Motors), Pat Brown (Founder, Impossible Foods), and other influential entrepreneurs from the biotech, fintech, and deeptech sectors.
The Honorable Mention recognition underscores KAIST’s commitment to global entrepreneurship education and the growing international visibility of the GESS program.
2025.07.29 View 499