GE
-
 Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body
Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans.
An adult human body comprises trillions of cells of more than 200 types. How a human develops from a single fertilized egg to a fully grown adult is a fundamental question in biomedical science. Due to the ethical challenges of performing studies on human embryos, however, the details of this process remain largely unknown.
To overcome these issues, the research team took a different approach. They analysed genetic mutations in cells taken from adult human post-mortem tissue. Specifically, they identified mutations that occur spontaneously in early developmental cell divisions. These mutations, also called genomic scars, act like unique genetic fingerprints that can be used to trace the embryonic development process.
The study, which looked at 334 single-cell colonies and 379 tissue samples from seven recently deceased human body donors, is the largest single-cell, whole-genome analysis carried out to date. The researchers examined the genomic scars of each individual in order to reconstruct their early embryonic cellular dynamics.
The result revealed several key characteristics of the human embryonic development process. Firstly, mutation rates are higher in the first cell division, but then decrease to approximately one mutation per cell during later cell division. Secondly, early cells contributed unequally to the development of the embryo in all informative donors, for example, at the two-cell stage, one of the cells always left more progeny cells than the other. The ratio of this was different from person to person, implying that the process varies between individuals and is not fully deterministic.
The researchers were also able to deduce the timing of when cells begin to differentiate into individual organ-specific cells. They found that within three days of fertilization, embryonic cells began to be distributed asymmetrically into tissues for the left and right sides of the body, followed by differentiation into three germ layers, and then differentiation into specific tissues and organs.
“It is an impressive scientific achievement that, within 20 years of the completion of human genome project, genomic technology has advanced to the extent that we are now able to accurately identify mutations in a single-cell genome,” said Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST. “This technology will enable us to track human embryogenesis at even higher resolutions in the future.”
The techniques used in this study could be used to improve our understanding of rare diseases caused by abnormalities in embryonic development, and to design new precision diagnostics and treatments for patients.
The research was completed in collaboration with Kyungpook National University Hospital, the Korea Institute of Science and Technology Information, Catholic University of Korea School of Medicine, Genome Insights Inc, and Immune Square Inc. This work was supported by the Suh Kyungbae Foundation, the Ministry of Health and Welfare of Korea, the National Research Foundastion of Korea.
-PublicationSeongyeol Park, Nanda Mali, Ryul Kim et al. ‘Clonal dynamics in early human embryogenesis inferred from somatic mutation’ Nature Online ahead of print, Aug. 25, 2021 (https://doi.org/10.1038/s41586-021-03786-8)
-ProfileProfessor Young Seok JuLab of Cancer Genomics (https://www.julab.kaist.ac.kr/)Graduate School of Medical Science and EngineeringKAIST
2021.08.31 View 10083
Genomic Data Reveals New Insights into Human Embryonic Development
KAIST researchers have used whole-genome sequencing to track the development from a single fertilized-egg to a human body
Genomic scientists at KAIST have revealed new insights into the process of human embryonic development using large-scale, whole-genome sequencing of cells and tissues from adult humans. The study, published in Nature on Aug.25, is the first to analyse somatic mutations in normal tissue across multiple organs within and between humans.
An adult human body comprises trillions of cells of more than 200 types. How a human develops from a single fertilized egg to a fully grown adult is a fundamental question in biomedical science. Due to the ethical challenges of performing studies on human embryos, however, the details of this process remain largely unknown.
To overcome these issues, the research team took a different approach. They analysed genetic mutations in cells taken from adult human post-mortem tissue. Specifically, they identified mutations that occur spontaneously in early developmental cell divisions. These mutations, also called genomic scars, act like unique genetic fingerprints that can be used to trace the embryonic development process.
The study, which looked at 334 single-cell colonies and 379 tissue samples from seven recently deceased human body donors, is the largest single-cell, whole-genome analysis carried out to date. The researchers examined the genomic scars of each individual in order to reconstruct their early embryonic cellular dynamics.
The result revealed several key characteristics of the human embryonic development process. Firstly, mutation rates are higher in the first cell division, but then decrease to approximately one mutation per cell during later cell division. Secondly, early cells contributed unequally to the development of the embryo in all informative donors, for example, at the two-cell stage, one of the cells always left more progeny cells than the other. The ratio of this was different from person to person, implying that the process varies between individuals and is not fully deterministic.
The researchers were also able to deduce the timing of when cells begin to differentiate into individual organ-specific cells. They found that within three days of fertilization, embryonic cells began to be distributed asymmetrically into tissues for the left and right sides of the body, followed by differentiation into three germ layers, and then differentiation into specific tissues and organs.
“It is an impressive scientific achievement that, within 20 years of the completion of human genome project, genomic technology has advanced to the extent that we are now able to accurately identify mutations in a single-cell genome,” said Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST. “This technology will enable us to track human embryogenesis at even higher resolutions in the future.”
The techniques used in this study could be used to improve our understanding of rare diseases caused by abnormalities in embryonic development, and to design new precision diagnostics and treatments for patients.
The research was completed in collaboration with Kyungpook National University Hospital, the Korea Institute of Science and Technology Information, Catholic University of Korea School of Medicine, Genome Insights Inc, and Immune Square Inc. This work was supported by the Suh Kyungbae Foundation, the Ministry of Health and Welfare of Korea, the National Research Foundastion of Korea.
-PublicationSeongyeol Park, Nanda Mali, Ryul Kim et al. ‘Clonal dynamics in early human embryogenesis inferred from somatic mutation’ Nature Online ahead of print, Aug. 25, 2021 (https://doi.org/10.1038/s41586-021-03786-8)
-ProfileProfessor Young Seok JuLab of Cancer Genomics (https://www.julab.kaist.ac.kr/)Graduate School of Medical Science and EngineeringKAIST
2021.08.31 View 10083 -
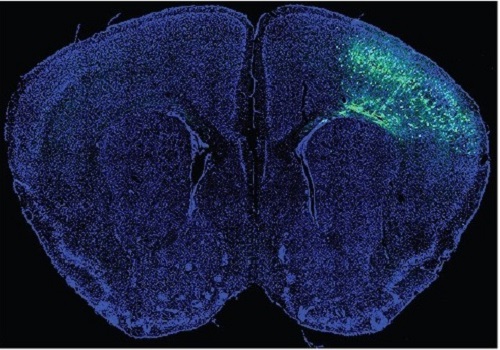 A Mechanism Underlying Most Common Cause of Epileptic Seizures Revealed
An interdisciplinary study shows that neurons carrying somatic mutations in MTOR can lead to focal epileptogenesis via non-cell-autonomous hyperexcitability of nearby nonmutated neurons
During fetal development, cells should migrate to the outer edge of the brain to form critical connections for information transfer and regulation in the body. When even a few cells fail to move to the correct location, the neurons become disorganized and this results in focal cortical dysplasia. This condition is the most common cause of seizures that cannot be controlled with medication in children and the second most common cause in adults.
Now, an interdisciplinary team studying neurogenetics, neural networks, and neurophysiology at KAIST has revealed how dysfunctions in even a small percentage of cells can cause disorder across the entire brain. They published their results on June 28 in Annals of Neurology.
The work builds on a previous finding, also by a KAIST scientists, who found that focal cortical dysplasia was caused by mutations in the cells involved in mTOR, a pathway that regulates signaling between neurons in the brain.
“Only 1 to 2% of neurons carrying mutations in the mTOR signaling pathway that regulates cell signaling in the brain have been found to include seizures in animal models of focal cortical dysplasia,” said Professor Jong-Woo Sohn from the Department of Biological Sciences. “The main challenge of this study was to explain how nearby non-mutated neurons are hyperexcitable.”
Initially, the researchers hypothesized that the mutated cells affected the number of excitatory and inhibitory synapses in all neurons, mutated or not. These neural gates can trigger or halt activity, respectively, in other neurons. Seizures are a result of extreme activity, called hyperexcitability. If the mutated cells upend the balance and result in more excitatory cells, the researchers thought, it made sense that the cells would be more susceptible to hyperexcitability and, as a result, seizures.
“Contrary to our expectations, the synaptic input balance was not changed in either the mutated or non-mutated neurons,” said Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering. “We turned our attention to a protein overproduced by mutated neurons.”
The protein is adenosine kinase, which lowers the concentration of adenosine. This naturally occurring compound is an anticonvulsant and works to relax vessels. In mice engineered to have focal cortical dysplasia, the researchers injected adenosine to replace the levels lowered by the protein. It worked and the neurons became less excitable.
“We demonstrated that augmentation of adenosine signaling could attenuate the excitability of non-mutated neurons,” said Professor Se-Bum Paik from the Department of Bio and Brain Engineering.
The effect on the non-mutated neurons was the surprising part, according to Paik. “The seizure-triggering hyperexcitability originated not in the mutation-carrying neurons, but instead in the nearby non-mutated neurons,” he said.
The mutated neurons excreted more adenosine kinase, reducing the adenosine levels in the local environment of all the cells. With less adenosine, the non-mutated neurons became hyperexcitable, leading to seizures.
“While we need further investigate into the relationship between the concentration of adenosine and the increased excitation of nearby neurons, our results support the medical use of drugs to activate adenosine signaling as a possible treatment pathway for focal cortical dysplasia,” Professor Lee said.
The Suh Kyungbae Foundation, the Korea Health Technology Research and Development Project, the Ministry of Health & Welfare, and the National Research Foundation in Korea funded this work.
-Publication:Koh, H.Y., Jang, J., Ju, S.H., Kim, R., Cho, G.-B., Kim, D.S., Sohn, J.-W., Paik, S.-B. and Lee, J.H. (2021), ‘Non–Cell Autonomous Epileptogenesis in Focal Cortical Dysplasia’ Annals of Neurology, 90: 285 299. (https://doi.org/10.1002/ana.26149)
-ProfileProfessor Jeong Ho Lee Translational Neurogenetics Labhttps://tnl.kaist.ac.kr/ Graduate School of Medical Science and Engineering KAIST
Professor Se-Bum Paik Visual System and Neural Network Laboratory http://vs.kaist.ac.kr/ Department of Bio and Brain EngineeringKAIST
Professor Jong-Woo Sohn Laboratory for Neurophysiology, https://sites.google.com/site/sohnlab2014/home Department of Biological SciencesKAIST
Dr. Hyun Yong Koh Translational Neurogenetics LabGraduate School of Medical Science and EngineeringKAIST
Dr. Jaeson Jang Ph.D.Visual System and Neural Network LaboratoryDepartment of Bio and Brain Engineering KAIST
Sang Hyeon Ju M.D.Laboratory for NeurophysiologyDepartment of Biological SciencesKAIST
2021.08.26 View 14733
A Mechanism Underlying Most Common Cause of Epileptic Seizures Revealed
An interdisciplinary study shows that neurons carrying somatic mutations in MTOR can lead to focal epileptogenesis via non-cell-autonomous hyperexcitability of nearby nonmutated neurons
During fetal development, cells should migrate to the outer edge of the brain to form critical connections for information transfer and regulation in the body. When even a few cells fail to move to the correct location, the neurons become disorganized and this results in focal cortical dysplasia. This condition is the most common cause of seizures that cannot be controlled with medication in children and the second most common cause in adults.
Now, an interdisciplinary team studying neurogenetics, neural networks, and neurophysiology at KAIST has revealed how dysfunctions in even a small percentage of cells can cause disorder across the entire brain. They published their results on June 28 in Annals of Neurology.
The work builds on a previous finding, also by a KAIST scientists, who found that focal cortical dysplasia was caused by mutations in the cells involved in mTOR, a pathway that regulates signaling between neurons in the brain.
“Only 1 to 2% of neurons carrying mutations in the mTOR signaling pathway that regulates cell signaling in the brain have been found to include seizures in animal models of focal cortical dysplasia,” said Professor Jong-Woo Sohn from the Department of Biological Sciences. “The main challenge of this study was to explain how nearby non-mutated neurons are hyperexcitable.”
Initially, the researchers hypothesized that the mutated cells affected the number of excitatory and inhibitory synapses in all neurons, mutated or not. These neural gates can trigger or halt activity, respectively, in other neurons. Seizures are a result of extreme activity, called hyperexcitability. If the mutated cells upend the balance and result in more excitatory cells, the researchers thought, it made sense that the cells would be more susceptible to hyperexcitability and, as a result, seizures.
“Contrary to our expectations, the synaptic input balance was not changed in either the mutated or non-mutated neurons,” said Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering. “We turned our attention to a protein overproduced by mutated neurons.”
The protein is adenosine kinase, which lowers the concentration of adenosine. This naturally occurring compound is an anticonvulsant and works to relax vessels. In mice engineered to have focal cortical dysplasia, the researchers injected adenosine to replace the levels lowered by the protein. It worked and the neurons became less excitable.
“We demonstrated that augmentation of adenosine signaling could attenuate the excitability of non-mutated neurons,” said Professor Se-Bum Paik from the Department of Bio and Brain Engineering.
The effect on the non-mutated neurons was the surprising part, according to Paik. “The seizure-triggering hyperexcitability originated not in the mutation-carrying neurons, but instead in the nearby non-mutated neurons,” he said.
The mutated neurons excreted more adenosine kinase, reducing the adenosine levels in the local environment of all the cells. With less adenosine, the non-mutated neurons became hyperexcitable, leading to seizures.
“While we need further investigate into the relationship between the concentration of adenosine and the increased excitation of nearby neurons, our results support the medical use of drugs to activate adenosine signaling as a possible treatment pathway for focal cortical dysplasia,” Professor Lee said.
The Suh Kyungbae Foundation, the Korea Health Technology Research and Development Project, the Ministry of Health & Welfare, and the National Research Foundation in Korea funded this work.
-Publication:Koh, H.Y., Jang, J., Ju, S.H., Kim, R., Cho, G.-B., Kim, D.S., Sohn, J.-W., Paik, S.-B. and Lee, J.H. (2021), ‘Non–Cell Autonomous Epileptogenesis in Focal Cortical Dysplasia’ Annals of Neurology, 90: 285 299. (https://doi.org/10.1002/ana.26149)
-ProfileProfessor Jeong Ho Lee Translational Neurogenetics Labhttps://tnl.kaist.ac.kr/ Graduate School of Medical Science and Engineering KAIST
Professor Se-Bum Paik Visual System and Neural Network Laboratory http://vs.kaist.ac.kr/ Department of Bio and Brain EngineeringKAIST
Professor Jong-Woo Sohn Laboratory for Neurophysiology, https://sites.google.com/site/sohnlab2014/home Department of Biological SciencesKAIST
Dr. Hyun Yong Koh Translational Neurogenetics LabGraduate School of Medical Science and EngineeringKAIST
Dr. Jaeson Jang Ph.D.Visual System and Neural Network LaboratoryDepartment of Bio and Brain Engineering KAIST
Sang Hyeon Ju M.D.Laboratory for NeurophysiologyDepartment of Biological SciencesKAIST
2021.08.26 View 14733 -
 A Study Reveals What Triggers Lung Damage during COVID-19
A longitudinal study of macrophages from SARS-CoV-2 infected lungs offers new insights into dynamic immunological changes
A KAIST immunology research team found that a specific subtype of macrophages that originated from blood monocytes plays a key role in the hyper-inflammatory response in SARS-CoV-2 infected lungs, by performing single-cell RNA sequencing of bronchoalveolar lavage fluid cells. This study provides new insights for understanding dynamic changes in immune responses to COVID-19.
In the early phase of COVID-19, SARS-CoV-2 infected lung tissue and the immediate defense system is activated. This early and fast response is called ‘innate immunity,’ provided by immune cells residing in lungs. Macrophages are major cell types of the innate immune system of the lungs, and newly differentiated macrophages originating from the bloodstream also contribute to early defenses against viruses.
Professor Su-Hyung Park and his collaborators investigated the quantitative and qualitative evaluation of immune responses in the lungs of SARS-CoV-2 infected ferrets. To overcome the limitations of research using patient-originated specimens, the researchers used a ferret infection model to obtain SARS-CoV-2 infected lungs sequentially with a defined time interval.
The researchers analyzed the 10 subtypes of macrophages during the five-day course of SARS-CoV-2 infection, and found that infiltrating macrophages originating from activated monocytes in the blood were key players for viral clearance as well as damaged lung tissue. Moreover, they found that the differentiation process of these inflammatory macrophages resembled the immune responses in the lung tissue of severe COVID-19 patients.
Currently, the research team is conducting a follow-up study to identify the dynamic changes in immune responses during the use of immunosuppressive agents to control hyper-inflammatory response called ‘cytokine storm’ in patients with COVID-19.
Dr. Jeong Seok Lee, the chief medical officer at Genome Insight Inc., explained, “Our analysis will enhance the understanding of the early features of COVID-19 immunity and provide a scientific background for the more precise use of immunosuppressive agents targeting specific macrophage subtypes.”
“This study is the first longitudinal study using sequentially obtained immune cells originating from SARS-CoV-2 infected lungs. The research describes the innate immune response to COVID-19 using single cell transcriptome data and enhances our understanding of the two phases of inflammatory responses,” Professor Park said.
This work was supported by the Ministry of Health and Welfare and KAIST, and was published in Nature Communications on July 28.
-PublicationSu-Hyung Park, Jeong Seok Lee, Su-Hyung Park et al. “Single-cell transcriptome of bronchoalverolar lavage fluid reveals sequential change of macrophages during SARS-CoV-2 infection in ferrets” Nature Communications (https://doi.org/10.1038/s41467-021-24807-0)
-ProfileProfessor Su-Hyung ParkLaboratory of Translational Immunology and Vaccinologyhttps://ltiv.kaist.ac.kr/
Graduate School of Medical Science and EngineeringKAIST
2021.08.04 View 13843
A Study Reveals What Triggers Lung Damage during COVID-19
A longitudinal study of macrophages from SARS-CoV-2 infected lungs offers new insights into dynamic immunological changes
A KAIST immunology research team found that a specific subtype of macrophages that originated from blood monocytes plays a key role in the hyper-inflammatory response in SARS-CoV-2 infected lungs, by performing single-cell RNA sequencing of bronchoalveolar lavage fluid cells. This study provides new insights for understanding dynamic changes in immune responses to COVID-19.
In the early phase of COVID-19, SARS-CoV-2 infected lung tissue and the immediate defense system is activated. This early and fast response is called ‘innate immunity,’ provided by immune cells residing in lungs. Macrophages are major cell types of the innate immune system of the lungs, and newly differentiated macrophages originating from the bloodstream also contribute to early defenses against viruses.
Professor Su-Hyung Park and his collaborators investigated the quantitative and qualitative evaluation of immune responses in the lungs of SARS-CoV-2 infected ferrets. To overcome the limitations of research using patient-originated specimens, the researchers used a ferret infection model to obtain SARS-CoV-2 infected lungs sequentially with a defined time interval.
The researchers analyzed the 10 subtypes of macrophages during the five-day course of SARS-CoV-2 infection, and found that infiltrating macrophages originating from activated monocytes in the blood were key players for viral clearance as well as damaged lung tissue. Moreover, they found that the differentiation process of these inflammatory macrophages resembled the immune responses in the lung tissue of severe COVID-19 patients.
Currently, the research team is conducting a follow-up study to identify the dynamic changes in immune responses during the use of immunosuppressive agents to control hyper-inflammatory response called ‘cytokine storm’ in patients with COVID-19.
Dr. Jeong Seok Lee, the chief medical officer at Genome Insight Inc., explained, “Our analysis will enhance the understanding of the early features of COVID-19 immunity and provide a scientific background for the more precise use of immunosuppressive agents targeting specific macrophage subtypes.”
“This study is the first longitudinal study using sequentially obtained immune cells originating from SARS-CoV-2 infected lungs. The research describes the innate immune response to COVID-19 using single cell transcriptome data and enhances our understanding of the two phases of inflammatory responses,” Professor Park said.
This work was supported by the Ministry of Health and Welfare and KAIST, and was published in Nature Communications on July 28.
-PublicationSu-Hyung Park, Jeong Seok Lee, Su-Hyung Park et al. “Single-cell transcriptome of bronchoalverolar lavage fluid reveals sequential change of macrophages during SARS-CoV-2 infection in ferrets” Nature Communications (https://doi.org/10.1038/s41467-021-24807-0)
-ProfileProfessor Su-Hyung ParkLaboratory of Translational Immunology and Vaccinologyhttps://ltiv.kaist.ac.kr/
Graduate School of Medical Science and EngineeringKAIST
2021.08.04 View 13843 -
 KPC4IR Helping to Create Global Standards for Virtual Transactions
KPC4IR will join the task force for the Global Implementation of Travel Rule Standards
The KAIST Policy Center for the Fourth Industrial Revolution (KPC4IR) will participate in a global initiative to create global standards for virtual asset transactions. As a member of the GI-TRUST (Global Implementation of Travel Rule Standards) task force, the KPC4IR will develop technical standards and relevant policies that support the global implementation of the travel rule for virtual assets in compliance with the recommendations of the Financial Action Task Force’s (FATF).
The FATF is an intergovernmental organization founded in 1989 by the G7 to develop policies to combat money laundering. In June 2019, the FATF extended its Recommendation 16, commonly known as the “travel rule,” to virtual asset services providers (VASPs), requiring both financial institutions and VASPs to aggregate information on the senders and recipients of wire transfers and exchange this information between parties to create a suitable audit trail.
According to the FATF’s recommendation and the G20’s support, jurisdictions, especially G20 member countries, have now applied the travel rule to their respective local laws. Korea also amended the Act on Reporting and Using Specified Financial Transaction Information in March 2020 to include virtual assets in their regulatory scope by March 2022.
The GI-TRUST task force will collaborate with global and local organizations developing travel rule technologies and offer a neutral assessment of proposed solutions. Their activities are aimed at standardizing related authentication protocols and security technologies that help VASPs comply with the travel rule.
The task force will also aid in the pilot testing of travel rule solutions for certain VASPs in Korea. Afterwards, the task force will report on the performance and reliability of the tested travel rule solutions for actual virtual asset transactions, in compliance with the FATF’s guidance.
Besides the KPC4IR, the GI-TRUST task force includes the Global Blockchain Business Council (GBBC), International Digital Asset Exchange Association (IDAXA), and Korea Blockchain Association (KBCA).
Director of the KPC4IR Professor So Young Kim will co-chair the task force. Professor Kim said their approach should be prudential in dealing with the regulations that rely on secure real-name data on top of the opposing governance style of pseudonymization, distribution, and recombination.
She explained, “KAIST has designed the co-evolution of technologies and institutions in conjunction with the global leaders’ groups such as the World Economic Forum and the EC Joint Research Center.”
She expects KAIST’s interdisciplinary, global cooperation to untie the entangled problem between regulations and technologies that obstruct future pathways.
2021.07.30 View 9878
KPC4IR Helping to Create Global Standards for Virtual Transactions
KPC4IR will join the task force for the Global Implementation of Travel Rule Standards
The KAIST Policy Center for the Fourth Industrial Revolution (KPC4IR) will participate in a global initiative to create global standards for virtual asset transactions. As a member of the GI-TRUST (Global Implementation of Travel Rule Standards) task force, the KPC4IR will develop technical standards and relevant policies that support the global implementation of the travel rule for virtual assets in compliance with the recommendations of the Financial Action Task Force’s (FATF).
The FATF is an intergovernmental organization founded in 1989 by the G7 to develop policies to combat money laundering. In June 2019, the FATF extended its Recommendation 16, commonly known as the “travel rule,” to virtual asset services providers (VASPs), requiring both financial institutions and VASPs to aggregate information on the senders and recipients of wire transfers and exchange this information between parties to create a suitable audit trail.
According to the FATF’s recommendation and the G20’s support, jurisdictions, especially G20 member countries, have now applied the travel rule to their respective local laws. Korea also amended the Act on Reporting and Using Specified Financial Transaction Information in March 2020 to include virtual assets in their regulatory scope by March 2022.
The GI-TRUST task force will collaborate with global and local organizations developing travel rule technologies and offer a neutral assessment of proposed solutions. Their activities are aimed at standardizing related authentication protocols and security technologies that help VASPs comply with the travel rule.
The task force will also aid in the pilot testing of travel rule solutions for certain VASPs in Korea. Afterwards, the task force will report on the performance and reliability of the tested travel rule solutions for actual virtual asset transactions, in compliance with the FATF’s guidance.
Besides the KPC4IR, the GI-TRUST task force includes the Global Blockchain Business Council (GBBC), International Digital Asset Exchange Association (IDAXA), and Korea Blockchain Association (KBCA).
Director of the KPC4IR Professor So Young Kim will co-chair the task force. Professor Kim said their approach should be prudential in dealing with the regulations that rely on secure real-name data on top of the opposing governance style of pseudonymization, distribution, and recombination.
She explained, “KAIST has designed the co-evolution of technologies and institutions in conjunction with the global leaders’ groups such as the World Economic Forum and the EC Joint Research Center.”
She expects KAIST’s interdisciplinary, global cooperation to untie the entangled problem between regulations and technologies that obstruct future pathways.
2021.07.30 View 9878 -
 Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 12854
Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 12854 -
 Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 14817
Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 14817 -
 Prof. Sang Wan Lee Selected for 2021 IBM Academic Award
Professor Sang Wan Lee from the Department of Bio and Brain Engineering was selected as the recipient of the 2021 IBM Global University Program Academic Award. The award recognizes individual faculty members whose emerging science and technology contains significant interest for universities and IBM.
Professor Lee, whose research focuses on artificial intelligence and computational neuroscience, won the award for his research proposal titled A Neuroscience-Inspired Approach for Metacognitive Reinforcement Learning. IBM provides a gift of $40,000 to the recipient’s institution in recognition of the selection of the project but not as a contract for services.
Professor Lee’s project aims to exploit the unique characteristics of human reinforcement learning. Specifically, he plans to examines the hypothesis that metacognition, a human’s ability to estimate their uncertainty level, serves to guide sample-efficient and near-optimal exploration, making it possible to achieve an optimal balance between model-based and model-free reinforcement learning.
He was also selected as the winner of the Google Research Award in 2016 and has been working with DeepMind and University College London to conduct basic research on decision-making brain science to establish a theory on frontal lobe meta-enhance learning.
"We plan to conduct joint research for utilizing brain-based artificial intelligence technology and frontal lobe meta-enhanced learning technology modeling in collaboration with an international research team including IBM, DeepMind, MIT, and Oxford,” Professor Lee said.
2021.06.25 View 13696
Prof. Sang Wan Lee Selected for 2021 IBM Academic Award
Professor Sang Wan Lee from the Department of Bio and Brain Engineering was selected as the recipient of the 2021 IBM Global University Program Academic Award. The award recognizes individual faculty members whose emerging science and technology contains significant interest for universities and IBM.
Professor Lee, whose research focuses on artificial intelligence and computational neuroscience, won the award for his research proposal titled A Neuroscience-Inspired Approach for Metacognitive Reinforcement Learning. IBM provides a gift of $40,000 to the recipient’s institution in recognition of the selection of the project but not as a contract for services.
Professor Lee’s project aims to exploit the unique characteristics of human reinforcement learning. Specifically, he plans to examines the hypothesis that metacognition, a human’s ability to estimate their uncertainty level, serves to guide sample-efficient and near-optimal exploration, making it possible to achieve an optimal balance between model-based and model-free reinforcement learning.
He was also selected as the winner of the Google Research Award in 2016 and has been working with DeepMind and University College London to conduct basic research on decision-making brain science to establish a theory on frontal lobe meta-enhance learning.
"We plan to conduct joint research for utilizing brain-based artificial intelligence technology and frontal lobe meta-enhanced learning technology modeling in collaboration with an international research team including IBM, DeepMind, MIT, and Oxford,” Professor Lee said.
2021.06.25 View 13696 -
 Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 11861
Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 11861 -
 ‘Urban Green Space Affects Citizens’ Happiness’
Study finds the relationship between green space, the economy, and happiness
A recent study revealed that as a city becomes more economically developed, its citizens’ happiness becomes more directly related to the area of urban green space.
A joint research project by Professor Meeyoung Cha of the School of Computing and her collaborators studied the relationship between green space and citizen happiness by analyzing big data from satellite images of 60 different countries.
Urban green space, including parks, gardens, and riversides not only provides aesthetic pleasure, but also positively affects our health by promoting physical activity and social interactions. Most of the previous research attempting to verify the correlation between urban green space and citizen happiness was based on few developed countries. Therefore, it was difficult to identify whether the positive effects of green space are global, or merely phenomena that depended on the economic state of the country. There have also been limitations in data collection, as it is difficult to visit each location or carry out investigations on a large scale based on aerial photographs.
The research team used data collected by Sentinel-2, a high-resolution satellite operated by the European Space Agency (ESA) to investigate 90 green spaces from 60 different countries around the world. The subjects of analysis were cities with the highest population densities (cities that contain at least 10% of the national population), and the images were obtained during the summer of each region for clarity. Images from the northern hemisphere were obtained between June and September of 2018, and those from the southern hemisphere were obtained between December of 2017 and February of 2018.
The areas of urban green space were then quantified and crossed with data from the World Happiness Report and GDP by country reported by the United Nations in 2018. Using these data, the relationships between green space, the economy, and citizen happiness were analyzed.
The results showed that in all cities, citizen happiness was positively correlated with the area of urban green space regardless of the country’s economic state. However, out of the 60 countries studied, the happiness index of the bottom 30 by GDP showed a stronger correlation with economic growth. In countries whose gross national income (GDP per capita) was higher than 38,000 USD, the area of green space acted as a more important factor affecting happiness than economic growth. Data from Seoul was analyzed to represent South Korea, and showed an increased happiness index with increased green areas compared to the past.
The authors point out their work has several policy-level implications. First, public green space should be made accessible to urban dwellers to enhance social support. If public safety in urban parks is not guaranteed, its positive role in social support and happiness may diminish. Also, the meaning of public safety may change; for example, ensuring biological safety will be a priority in keeping urban parks accessible during the COVID-19 pandemic.
Second, urban planning for public green space is needed for both developed and developing countries. As it is challenging or nearly impossible to secure land for green space after the area is developed, urban planning for parks and green space should be considered in developing economies where new cities and suburban areas are rapidly expanding.
Third, recent climate changes can present substantial difficulty in sustaining urban green space. Extreme events such as wildfires, floods, droughts, and cold waves could endanger urban forests while global warming could conversely accelerate tree growth in cities due to the urban heat island effect. Thus, more attention must be paid to predict climate changes and discovering their impact on the maintenance of urban green space.
“There has recently been an increase in the number of studies using big data from satellite images to solve social conundrums,” said Professor Cha. “The tool developed for this investigation can also be used to quantify the area of aquatic environments like lakes and the seaside, and it will now be possible to analyze the relationship between citizen happiness and aquatic environments in future studies,” she added.
Professor Woo Sung Jung from POSTECH and Professor Donghee Wohn from the New Jersey Institute of Technology also joined this research. It was reported in the online issue of EPJ Data Science on May 30.
-PublicationOh-Hyun Kwon, Inho Hong, Jeasurk Yang, Donghee Y. Wohn, Woo-Sung Jung, andMeeyoung Cha, 2021. Urban green space and happiness in developed countries. EPJ Data Science. DOI: https://doi.org/10.1140/epjds/s13688-021-00278-7
-ProfileProfessor Meeyoung ChaData Science Labhttps://ds.ibs.re.kr/
School of Computing
KAIST
2021.06.21 View 13180
‘Urban Green Space Affects Citizens’ Happiness’
Study finds the relationship between green space, the economy, and happiness
A recent study revealed that as a city becomes more economically developed, its citizens’ happiness becomes more directly related to the area of urban green space.
A joint research project by Professor Meeyoung Cha of the School of Computing and her collaborators studied the relationship between green space and citizen happiness by analyzing big data from satellite images of 60 different countries.
Urban green space, including parks, gardens, and riversides not only provides aesthetic pleasure, but also positively affects our health by promoting physical activity and social interactions. Most of the previous research attempting to verify the correlation between urban green space and citizen happiness was based on few developed countries. Therefore, it was difficult to identify whether the positive effects of green space are global, or merely phenomena that depended on the economic state of the country. There have also been limitations in data collection, as it is difficult to visit each location or carry out investigations on a large scale based on aerial photographs.
The research team used data collected by Sentinel-2, a high-resolution satellite operated by the European Space Agency (ESA) to investigate 90 green spaces from 60 different countries around the world. The subjects of analysis were cities with the highest population densities (cities that contain at least 10% of the national population), and the images were obtained during the summer of each region for clarity. Images from the northern hemisphere were obtained between June and September of 2018, and those from the southern hemisphere were obtained between December of 2017 and February of 2018.
The areas of urban green space were then quantified and crossed with data from the World Happiness Report and GDP by country reported by the United Nations in 2018. Using these data, the relationships between green space, the economy, and citizen happiness were analyzed.
The results showed that in all cities, citizen happiness was positively correlated with the area of urban green space regardless of the country’s economic state. However, out of the 60 countries studied, the happiness index of the bottom 30 by GDP showed a stronger correlation with economic growth. In countries whose gross national income (GDP per capita) was higher than 38,000 USD, the area of green space acted as a more important factor affecting happiness than economic growth. Data from Seoul was analyzed to represent South Korea, and showed an increased happiness index with increased green areas compared to the past.
The authors point out their work has several policy-level implications. First, public green space should be made accessible to urban dwellers to enhance social support. If public safety in urban parks is not guaranteed, its positive role in social support and happiness may diminish. Also, the meaning of public safety may change; for example, ensuring biological safety will be a priority in keeping urban parks accessible during the COVID-19 pandemic.
Second, urban planning for public green space is needed for both developed and developing countries. As it is challenging or nearly impossible to secure land for green space after the area is developed, urban planning for parks and green space should be considered in developing economies where new cities and suburban areas are rapidly expanding.
Third, recent climate changes can present substantial difficulty in sustaining urban green space. Extreme events such as wildfires, floods, droughts, and cold waves could endanger urban forests while global warming could conversely accelerate tree growth in cities due to the urban heat island effect. Thus, more attention must be paid to predict climate changes and discovering their impact on the maintenance of urban green space.
“There has recently been an increase in the number of studies using big data from satellite images to solve social conundrums,” said Professor Cha. “The tool developed for this investigation can also be used to quantify the area of aquatic environments like lakes and the seaside, and it will now be possible to analyze the relationship between citizen happiness and aquatic environments in future studies,” she added.
Professor Woo Sung Jung from POSTECH and Professor Donghee Wohn from the New Jersey Institute of Technology also joined this research. It was reported in the online issue of EPJ Data Science on May 30.
-PublicationOh-Hyun Kwon, Inho Hong, Jeasurk Yang, Donghee Y. Wohn, Woo-Sung Jung, andMeeyoung Cha, 2021. Urban green space and happiness in developed countries. EPJ Data Science. DOI: https://doi.org/10.1140/epjds/s13688-021-00278-7
-ProfileProfessor Meeyoung ChaData Science Labhttps://ds.ibs.re.kr/
School of Computing
KAIST
2021.06.21 View 13180 -
 Identification of How Chemotherapy Drug Works Could Deliver Personalized Cancer Treatment
The chemotherapy drug decitabine is commonly used to treat patients with blood cancers, but its response rate is somewhat low. Researchers have now identified why this is the case, opening the door to more personalized cancer therapies for those with these types of cancers, and perhaps further afield.
Researchers have identified the genetic and molecular mechanisms within cells that make the chemotherapy drug decitabine—used to treat patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) —work for some patients but not others. The findings should assist clinicians in developing more patient-specific treatment strategies.
The findings were published in the Proceedings of the National Academies of Science on March 30.
The chemotherapy drug decitabine, also known by its brand name Dacogen, works by modifying our DNA that in turn switches on genes that stop the cancer cells from growing and replicating. However, decitabine’s response rate is somewhat low (showing improvement in just 30-35% of patients), which leaves something of a mystery as to why it works well for some patients but not for others. To find out why this happens, researchers from the KAIST investigated the molecular mediators that are involved with regulating the effects of the drug.
Decitabine works to activate the production of endogenous retroviruses (ERVs), which in turn induces an immune response. ERVs are viruses that long ago inserted dormant copies of themselves into the human genome. Decitabine in essence, ‘reactivates’ these viral elements and produces double-stranded RNAs (dsRNAs) that the immune system views as a foreign body.
“However, the mechanisms involved in this process, in particular how production and transport of these ERV dsRNAs were regulated within the cell were understudied,” said corresponding author Yoosik Kim, professor in the Department of Chemical and Biomolecular Engineering at KAIST.
“So to explain why decitabine works in some patients but not others, we investigated what these molecular mechanisms were,” added Kim.
To do so, the researchers used image-based RNA interference (RNAi) screening. This is a relatively new technique in which specific sequences within a genome are knocked out of action or “downregulated.” Large-scale screening, which can be performed in cultured cells or within live organisms, works to investigate the function of different genes. The KAIST researchers collaborated with the Institut Pasteur Korea to analyze the effect of downregulating genes that recognize ERV dsRNAs and could be involved in the cellular response to decitabine.
From these initial screening results, they performed an even more detailed downregulation screening analysis. Through the screening, they were able to identify two particular gene sequences involved in the production of an RNA-binding protein called Staufen1 and the production of a strand of RNA that does not in turn produce any proteins called TINCR that play a key regulatory role in response to the drug. Staufen1 binds directly to dsRNAs and stabilizes them in concert with the TINCR.
If a patient is not producing sufficient Staufen1 and TINCR, then the dsRNA viral mimics quickly degrade before the immune system can spot them. And, crucially for cancer therapy, this means that patients with lower expression (activation) of these sequences will show inferior response to decitabine. Indeed, the researchers confirmed that MDS/AML patients with low Staufen1 and TINCR expression did not benefit from decitabine therapy.
“We can now isolate patients who will not benefit from the therapy and direct them to a different type of therapy,” said first author Yongsuk Ku. “This serves as an important step toward developing a patient-specific treatment cancer strategy.”
As the researchers used patient samples taken from bone marrow, the next step will be to try to develop a testing method that can identify the problem from just blood samples, which are much easier to acquire from patients.
The team plans to investigate if the analysis can be extended to patients with solid tumors in addition to those with blood cancers.
-Profile
Professor Yoosik Kim
https://qcbio.kaist.ac.kr/
Department of Chemical and Biomolecular Engineering
KAIST
-Publication
Noncanonical immune response to the inhibition of DNA methylation by Staufen1 via stabilization of endogenous retrovirus RNAs, PNAS
2021.05.24 View 11645
Identification of How Chemotherapy Drug Works Could Deliver Personalized Cancer Treatment
The chemotherapy drug decitabine is commonly used to treat patients with blood cancers, but its response rate is somewhat low. Researchers have now identified why this is the case, opening the door to more personalized cancer therapies for those with these types of cancers, and perhaps further afield.
Researchers have identified the genetic and molecular mechanisms within cells that make the chemotherapy drug decitabine—used to treat patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) —work for some patients but not others. The findings should assist clinicians in developing more patient-specific treatment strategies.
The findings were published in the Proceedings of the National Academies of Science on March 30.
The chemotherapy drug decitabine, also known by its brand name Dacogen, works by modifying our DNA that in turn switches on genes that stop the cancer cells from growing and replicating. However, decitabine’s response rate is somewhat low (showing improvement in just 30-35% of patients), which leaves something of a mystery as to why it works well for some patients but not for others. To find out why this happens, researchers from the KAIST investigated the molecular mediators that are involved with regulating the effects of the drug.
Decitabine works to activate the production of endogenous retroviruses (ERVs), which in turn induces an immune response. ERVs are viruses that long ago inserted dormant copies of themselves into the human genome. Decitabine in essence, ‘reactivates’ these viral elements and produces double-stranded RNAs (dsRNAs) that the immune system views as a foreign body.
“However, the mechanisms involved in this process, in particular how production and transport of these ERV dsRNAs were regulated within the cell were understudied,” said corresponding author Yoosik Kim, professor in the Department of Chemical and Biomolecular Engineering at KAIST.
“So to explain why decitabine works in some patients but not others, we investigated what these molecular mechanisms were,” added Kim.
To do so, the researchers used image-based RNA interference (RNAi) screening. This is a relatively new technique in which specific sequences within a genome are knocked out of action or “downregulated.” Large-scale screening, which can be performed in cultured cells or within live organisms, works to investigate the function of different genes. The KAIST researchers collaborated with the Institut Pasteur Korea to analyze the effect of downregulating genes that recognize ERV dsRNAs and could be involved in the cellular response to decitabine.
From these initial screening results, they performed an even more detailed downregulation screening analysis. Through the screening, they were able to identify two particular gene sequences involved in the production of an RNA-binding protein called Staufen1 and the production of a strand of RNA that does not in turn produce any proteins called TINCR that play a key regulatory role in response to the drug. Staufen1 binds directly to dsRNAs and stabilizes them in concert with the TINCR.
If a patient is not producing sufficient Staufen1 and TINCR, then the dsRNA viral mimics quickly degrade before the immune system can spot them. And, crucially for cancer therapy, this means that patients with lower expression (activation) of these sequences will show inferior response to decitabine. Indeed, the researchers confirmed that MDS/AML patients with low Staufen1 and TINCR expression did not benefit from decitabine therapy.
“We can now isolate patients who will not benefit from the therapy and direct them to a different type of therapy,” said first author Yongsuk Ku. “This serves as an important step toward developing a patient-specific treatment cancer strategy.”
As the researchers used patient samples taken from bone marrow, the next step will be to try to develop a testing method that can identify the problem from just blood samples, which are much easier to acquire from patients.
The team plans to investigate if the analysis can be extended to patients with solid tumors in addition to those with blood cancers.
-Profile
Professor Yoosik Kim
https://qcbio.kaist.ac.kr/
Department of Chemical and Biomolecular Engineering
KAIST
-Publication
Noncanonical immune response to the inhibition of DNA methylation by Staufen1 via stabilization of endogenous retrovirus RNAs, PNAS
2021.05.24 View 11645 -
 Acoustic Graphene Plasmons Study Paves Way for Optoelectronic Applications
- The first images of mid-infrared optical waves compressed 1,000 times captured using a highly sensitive scattering-type scanning near-field optical microscope. -
KAIST researchers and their collaborators at home and abroad have successfully demonstrated a new methodology for direct near-field optical imaging of acoustic graphene plasmon fields. This strategy will provide a breakthrough for the practical applications of acoustic graphene plasmon platforms in next-generation, high-performance, graphene-based optoelectronic devices with enhanced light-matter interactions and lower propagation loss.
It was recently demonstrated that ‘graphene plasmons’ – collective oscillations of free electrons in graphene coupled to electromagnetic waves of light – can be used to trap and compress optical waves inside a very thin dielectric layer separating graphene from a metallic sheet. In such a configuration, graphene’s conduction electrons are “reflected” in the metal, so when the light waves “push” the electrons in graphene, their image charges in metal also start to oscillate. This new type of collective electronic oscillation mode is called ‘acoustic graphene plasmon (AGP)’.
The existence of AGP could previously be observed only via indirect methods such as far-field infrared spectroscopy and photocurrent mapping. This indirect observation was the price that researchers had to pay for the strong compression of optical waves inside nanometer-thin structures. It was believed that the intensity of electromagnetic fields outside the device was insufficient for direct near-field optical imaging of AGP.
Challenged by these limitations, three research groups combined their efforts to bring together a unique experimental technique using advanced nanofabrication methods. Their findings were published in Nature Communications on February 19.
A KAIST research team led by Professor Min Seok Jang from the School of Electrical Engineering used a highly sensitive scattering-type scanning near-field optical microscope (s-SNOM) to directly measure the optical fields of the AGP waves propagating in a nanometer-thin waveguide, visualizing thousand-fold compression of mid-infrared light for the first time.
Professor Jang and a post-doc researcher in his group, Sergey G. Menabde, successfully obtained direct images of AGP waves by taking advantage of their rapidly decaying yet always present electric field above graphene. They showed that AGPs are detectable even when most of their energy is flowing inside the dielectric below the graphene.
This became possible due to the ultra-smooth surfaces inside the nano-waveguides where plasmonic waves can propagate at longer distances. The AGP mode probed by the researchers was up to 2.3 times more confined and exhibited a 1.4 times higher figure of merit in terms of the normalized propagation length compared to the graphene surface plasmon under similar conditions.
These ultra-smooth nanostructures of the waveguides used in the experiment were created using a template-stripping method by Professor Sang-Hyun Oh and a post-doc researcher, In-Ho Lee, from the Department of Electrical and Computer Engineering at the University of Minnesota.
Professor Young Hee Lee and his researchers at the Center for Integrated Nanostructure Physics (CINAP) of the Institute of Basic Science (IBS) at Sungkyunkwan University synthesized the graphene with a monocrystalline structure, and this high-quality, large-area graphene enabled low-loss plasmonic propagation.
The chemical and physical properties of many important organic molecules can be detected and evaluated by their absorption signatures in the mid-infrared spectrum. However, conventional detection methods require a large number of molecules for successful detection, whereas the ultra-compressed AGP fields can provide strong light-matter interactions at the microscopic level, thus significantly improving the detection sensitivity down to a single molecule.
Furthermore, the study conducted by Professor Jang and the team demonstrated that the mid-infrared AGPs are inherently less sensitive to losses in graphene due to their fields being mostly confined within the dielectric. The research team’s reported results suggest that AGPs could become a promising platform for electrically tunable graphene-based optoelectronic devices that typically suffer from higher absorption rates in graphene such as metasurfaces, optical switches, photovoltaics, and other optoelectronic applications operating at infrared frequencies.
Professor Jang said, “Our research revealed that the ultra-compressed electromagnetic fields of acoustic graphene plasmons can be directly accessed through near-field optical microscopy methods. I hope this realization will motivate other researchers to apply AGPs to various problems where strong light-matter interactions and lower propagation loss are needed.”
This research was primarily funded by the Samsung Research Funding & Incubation Center of Samsung Electronics. The National Research Foundation of Korea (NRF), the U.S. National Science Foundation (NSF), Samsung Global Research Outreach (GRO) Program, and Institute for Basic Science of Korea (IBS) also supported the work.
Publication:
Menabde, S. G., et al. (2021) Real-space imaging of acoustic plasmons in large-area graphene grown by chemical vapor deposition. Nature Communications 12, Article No. 938. Available online at https://doi.org/10.1038/s41467-021-21193-5
Profile:
Min Seok Jang, MS, PhD
Associate Professorjang.minseok@kaist.ac.krhttp://jlab.kaist.ac.kr/
Min Seok Jang Research GroupSchool of Electrical Engineering
http://kaist.ac.kr/en/Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2021.03.16 View 15907
Acoustic Graphene Plasmons Study Paves Way for Optoelectronic Applications
- The first images of mid-infrared optical waves compressed 1,000 times captured using a highly sensitive scattering-type scanning near-field optical microscope. -
KAIST researchers and their collaborators at home and abroad have successfully demonstrated a new methodology for direct near-field optical imaging of acoustic graphene plasmon fields. This strategy will provide a breakthrough for the practical applications of acoustic graphene plasmon platforms in next-generation, high-performance, graphene-based optoelectronic devices with enhanced light-matter interactions and lower propagation loss.
It was recently demonstrated that ‘graphene plasmons’ – collective oscillations of free electrons in graphene coupled to electromagnetic waves of light – can be used to trap and compress optical waves inside a very thin dielectric layer separating graphene from a metallic sheet. In such a configuration, graphene’s conduction electrons are “reflected” in the metal, so when the light waves “push” the electrons in graphene, their image charges in metal also start to oscillate. This new type of collective electronic oscillation mode is called ‘acoustic graphene plasmon (AGP)’.
The existence of AGP could previously be observed only via indirect methods such as far-field infrared spectroscopy and photocurrent mapping. This indirect observation was the price that researchers had to pay for the strong compression of optical waves inside nanometer-thin structures. It was believed that the intensity of electromagnetic fields outside the device was insufficient for direct near-field optical imaging of AGP.
Challenged by these limitations, three research groups combined their efforts to bring together a unique experimental technique using advanced nanofabrication methods. Their findings were published in Nature Communications on February 19.
A KAIST research team led by Professor Min Seok Jang from the School of Electrical Engineering used a highly sensitive scattering-type scanning near-field optical microscope (s-SNOM) to directly measure the optical fields of the AGP waves propagating in a nanometer-thin waveguide, visualizing thousand-fold compression of mid-infrared light for the first time.
Professor Jang and a post-doc researcher in his group, Sergey G. Menabde, successfully obtained direct images of AGP waves by taking advantage of their rapidly decaying yet always present electric field above graphene. They showed that AGPs are detectable even when most of their energy is flowing inside the dielectric below the graphene.
This became possible due to the ultra-smooth surfaces inside the nano-waveguides where plasmonic waves can propagate at longer distances. The AGP mode probed by the researchers was up to 2.3 times more confined and exhibited a 1.4 times higher figure of merit in terms of the normalized propagation length compared to the graphene surface plasmon under similar conditions.
These ultra-smooth nanostructures of the waveguides used in the experiment were created using a template-stripping method by Professor Sang-Hyun Oh and a post-doc researcher, In-Ho Lee, from the Department of Electrical and Computer Engineering at the University of Minnesota.
Professor Young Hee Lee and his researchers at the Center for Integrated Nanostructure Physics (CINAP) of the Institute of Basic Science (IBS) at Sungkyunkwan University synthesized the graphene with a monocrystalline structure, and this high-quality, large-area graphene enabled low-loss plasmonic propagation.
The chemical and physical properties of many important organic molecules can be detected and evaluated by their absorption signatures in the mid-infrared spectrum. However, conventional detection methods require a large number of molecules for successful detection, whereas the ultra-compressed AGP fields can provide strong light-matter interactions at the microscopic level, thus significantly improving the detection sensitivity down to a single molecule.
Furthermore, the study conducted by Professor Jang and the team demonstrated that the mid-infrared AGPs are inherently less sensitive to losses in graphene due to their fields being mostly confined within the dielectric. The research team’s reported results suggest that AGPs could become a promising platform for electrically tunable graphene-based optoelectronic devices that typically suffer from higher absorption rates in graphene such as metasurfaces, optical switches, photovoltaics, and other optoelectronic applications operating at infrared frequencies.
Professor Jang said, “Our research revealed that the ultra-compressed electromagnetic fields of acoustic graphene plasmons can be directly accessed through near-field optical microscopy methods. I hope this realization will motivate other researchers to apply AGPs to various problems where strong light-matter interactions and lower propagation loss are needed.”
This research was primarily funded by the Samsung Research Funding & Incubation Center of Samsung Electronics. The National Research Foundation of Korea (NRF), the U.S. National Science Foundation (NSF), Samsung Global Research Outreach (GRO) Program, and Institute for Basic Science of Korea (IBS) also supported the work.
Publication:
Menabde, S. G., et al. (2021) Real-space imaging of acoustic plasmons in large-area graphene grown by chemical vapor deposition. Nature Communications 12, Article No. 938. Available online at https://doi.org/10.1038/s41467-021-21193-5
Profile:
Min Seok Jang, MS, PhD
Associate Professorjang.minseok@kaist.ac.krhttp://jlab.kaist.ac.kr/
Min Seok Jang Research GroupSchool of Electrical Engineering
http://kaist.ac.kr/en/Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2021.03.16 View 15907 -
 Rare Mutations May Have Big Impact on Schizophrenia Pathology
- Somatic mutations found only in brain cells disrupt synaptic function. -
Schizophrenia is a neurodevelopmental disorder that disrupts brain activity, producing hallucinations, delusions, and other cognitive disturbances. Researchers have long searched for genetic influences in the disease, but genetic mutations have been identified in only a small fraction—fewer than a quarter—of sequenced patients. Now a study shows that “somatic” gene mutations in brain cells could account for some of the disease’s neuropathology.
The results of the study, led by Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering in collaboration with the Stanley Medical Research Institute in the US, appeared in Biological Psychiatry.
Traditional genetic mutations, called germline mutations, occur in sperm or egg cells and are passed on to offspring by their parents. Somatic mutations, in contrast, occur in an embryo after fertilization, and they can show up throughout the body or in isolated pockets of tissues, making them much harder to detect from blood or saliva samples, which are typically used for such sequencing studies.
Recently, more-advanced genetic sequencing techniques have allowed researchers to detect somatic mutations and studies have shown that even mutations present at very low levels can have functional consequences. A previous study hinted that brain somatic mutations were associated with schizophrenia, but it was not powerful enough to cement an association between brain somatic mutations and schizophrenia.
In the current study, the researchers used deep whole-exome sequencing to determine the genetic code of all exomes, the parts of genes that encode proteins. The scientists sequenced postmortem samples from brain, liver, spleen, or heart tissue of 27 people with schizophrenia and 31 control participants allowing them to compare the sequences in the two tissues. Using a powerful analytic technique, the team identified an average of 4.9 somatic single-nucleotide variants, or mutations, in brain samples from people with schizophrenia, and 5.6 somatic single-nucleotide variants in brain samples from control subjects.
Although there were no significant quantitative differences in somatic single-nucleotide variants between schizophrenia and control tissue samples, the researchers found that the mutations in schizophrenia patients were found in genes already associated with schizophrenia. Of the germline mutations that had previously been associated with schizophrenia, the genes affected encode proteins associated with synaptic neural communication, particularly in a brain region called the dorsolateral prefrontal cortex.
In the new analysis, the researchers determined which proteins might be affected by the newly identified somatic mutations. Remarkably, a protein called GRIN2B emerged as highly affected and two patients with schizophrenia carried somatic mutations on the GRIN2B gene itself. GRIN2B is a protein component of NMDA-type glutamate receptors, which are critical for neural signaling. Faulty glutamate receptors have long been suspected of contributing to schizophrenia pathology; GRIN2B ranks among the most-studied genes in schizophrenia. The somatic mutations identified in the study had a variant allele frequency of only ~1%, indicating that the mutations were rare among brain cells as a whole. Nevertheless, they have the potential to create widespread cortical dysfunction.
Professor Lee said, “Besides the comprehensive genetic analysis of brain-only mutations in postmortem tissues from schizophrenia patients, this study experimentally showed the biological consequence of identified somatic mutations, which led to neuronal abnormalities associated with schizophrenia. Thus, this study suggests that brain somatic mutations can be a hidden major contributor to schizophrenia and provides new insights into the molecular genetic architecture of schizophrenia.
John Krystal, MD, editor of Biological Psychiatry, said of the work, "The genetics of schizophrenia has received intensive study for several decades. Now a new possibility emerges, that in some cases, mutations in the DNA of brain cells contributes to the biology of schizophrenia. Remarkably this new biology points to an old schizophrenia story: NMDA glutamate receptor dysfunction. Perhaps the path through which somatic mutations contribute to schizophrenia converges with other sources of abnormalities in glutamate signaling in this disorder."
Professor Lee and the team next want to assess the functional consequences of the somatic mutations. Because of the location of the GRIN2B mutations found in schizophrenia patients, the researchers hypothesized that they might interfere with the receptors’ localization on neurons. Experiments on the cortical neurons of mice showed that the mutations indeed disrupted the receptors’ usual localization to dendrites, the “listening” ends of neurons, which in turn prevented the formation of normal synapses in the neurons. This finding suggests that the somatic mutations could disrupt neural communication, contributing to schizophrenia pathology.
- Profile:
Professor Jeong Ho Lee
Translational Neurogenetics Laboratory ( https://tnl.kaist.ac.kr/)
The Graduate School of Medical Science and Engineering
KAIST
(END)
2021.03.11 View 8751
Rare Mutations May Have Big Impact on Schizophrenia Pathology
- Somatic mutations found only in brain cells disrupt synaptic function. -
Schizophrenia is a neurodevelopmental disorder that disrupts brain activity, producing hallucinations, delusions, and other cognitive disturbances. Researchers have long searched for genetic influences in the disease, but genetic mutations have been identified in only a small fraction—fewer than a quarter—of sequenced patients. Now a study shows that “somatic” gene mutations in brain cells could account for some of the disease’s neuropathology.
The results of the study, led by Professor Jeong Ho Lee at the Graduate School of Medical Science and Engineering in collaboration with the Stanley Medical Research Institute in the US, appeared in Biological Psychiatry.
Traditional genetic mutations, called germline mutations, occur in sperm or egg cells and are passed on to offspring by their parents. Somatic mutations, in contrast, occur in an embryo after fertilization, and they can show up throughout the body or in isolated pockets of tissues, making them much harder to detect from blood or saliva samples, which are typically used for such sequencing studies.
Recently, more-advanced genetic sequencing techniques have allowed researchers to detect somatic mutations and studies have shown that even mutations present at very low levels can have functional consequences. A previous study hinted that brain somatic mutations were associated with schizophrenia, but it was not powerful enough to cement an association between brain somatic mutations and schizophrenia.
In the current study, the researchers used deep whole-exome sequencing to determine the genetic code of all exomes, the parts of genes that encode proteins. The scientists sequenced postmortem samples from brain, liver, spleen, or heart tissue of 27 people with schizophrenia and 31 control participants allowing them to compare the sequences in the two tissues. Using a powerful analytic technique, the team identified an average of 4.9 somatic single-nucleotide variants, or mutations, in brain samples from people with schizophrenia, and 5.6 somatic single-nucleotide variants in brain samples from control subjects.
Although there were no significant quantitative differences in somatic single-nucleotide variants between schizophrenia and control tissue samples, the researchers found that the mutations in schizophrenia patients were found in genes already associated with schizophrenia. Of the germline mutations that had previously been associated with schizophrenia, the genes affected encode proteins associated with synaptic neural communication, particularly in a brain region called the dorsolateral prefrontal cortex.
In the new analysis, the researchers determined which proteins might be affected by the newly identified somatic mutations. Remarkably, a protein called GRIN2B emerged as highly affected and two patients with schizophrenia carried somatic mutations on the GRIN2B gene itself. GRIN2B is a protein component of NMDA-type glutamate receptors, which are critical for neural signaling. Faulty glutamate receptors have long been suspected of contributing to schizophrenia pathology; GRIN2B ranks among the most-studied genes in schizophrenia. The somatic mutations identified in the study had a variant allele frequency of only ~1%, indicating that the mutations were rare among brain cells as a whole. Nevertheless, they have the potential to create widespread cortical dysfunction.
Professor Lee said, “Besides the comprehensive genetic analysis of brain-only mutations in postmortem tissues from schizophrenia patients, this study experimentally showed the biological consequence of identified somatic mutations, which led to neuronal abnormalities associated with schizophrenia. Thus, this study suggests that brain somatic mutations can be a hidden major contributor to schizophrenia and provides new insights into the molecular genetic architecture of schizophrenia.
John Krystal, MD, editor of Biological Psychiatry, said of the work, "The genetics of schizophrenia has received intensive study for several decades. Now a new possibility emerges, that in some cases, mutations in the DNA of brain cells contributes to the biology of schizophrenia. Remarkably this new biology points to an old schizophrenia story: NMDA glutamate receptor dysfunction. Perhaps the path through which somatic mutations contribute to schizophrenia converges with other sources of abnormalities in glutamate signaling in this disorder."
Professor Lee and the team next want to assess the functional consequences of the somatic mutations. Because of the location of the GRIN2B mutations found in schizophrenia patients, the researchers hypothesized that they might interfere with the receptors’ localization on neurons. Experiments on the cortical neurons of mice showed that the mutations indeed disrupted the receptors’ usual localization to dendrites, the “listening” ends of neurons, which in turn prevented the formation of normal synapses in the neurons. This finding suggests that the somatic mutations could disrupt neural communication, contributing to schizophrenia pathology.
- Profile:
Professor Jeong Ho Lee
Translational Neurogenetics Laboratory ( https://tnl.kaist.ac.kr/)
The Graduate School of Medical Science and Engineering
KAIST
(END)
2021.03.11 View 8751