materials
-
 KAIST College of Life Sciences and Bioengineering Signs MOU with Harvard
KAIST’s College of Life Sciences and Bioengineering recently signed a memorandum of understanding (MOU) with Harvard University’s Center for Brain Science on July 20, which will allow for joint research and exchange in researchers between the two institutions.
Headed by Director Kenneth Blum, Harvard’s Center for Brain Science leads the world in brain-related research. The new MOU will allow for research cooperation, exchanges of professors, researchers, and students, joint usage of infrastructure and research materials, and finally, sharing of research assignments.
The Dean of the College of Life Sciences and Bioengineering Sang Yup Lee, who concerted efforts to form the MOU said, “This agreement will bring together two of the world’s leading brain-related research teams, and I hope that combining their expertise will bring great advances in brain science and engineering.
KAIST’s College of Life Science and Bioengineering, which is known for its creative interdisciplinary research, is producing exemplary research results in the field of brain science from its Biological Sciences and Bio and Brain Engineering departments.
In addition to cooperation with Harvard, KAIST has also formed partnerships with Emory University, Japan’s RIKEN Brain Institute, and Germany’s Max Planck Institute. Not only does it have a worldwide network pertaining to brain research, but KAIST has also engaged in cooperative research with prominent domestic institutions such as, Asan Medical Center, the Korea Research Institute of Bioscience and Biotechnology, the Korea Research Institute of Standards and Science, and the SK Corporation. Through these connections, KAIST has managed to lead in mutually cooperative brain interdisciplinary research.
2009.08.10 View 20890
KAIST College of Life Sciences and Bioengineering Signs MOU with Harvard
KAIST’s College of Life Sciences and Bioengineering recently signed a memorandum of understanding (MOU) with Harvard University’s Center for Brain Science on July 20, which will allow for joint research and exchange in researchers between the two institutions.
Headed by Director Kenneth Blum, Harvard’s Center for Brain Science leads the world in brain-related research. The new MOU will allow for research cooperation, exchanges of professors, researchers, and students, joint usage of infrastructure and research materials, and finally, sharing of research assignments.
The Dean of the College of Life Sciences and Bioengineering Sang Yup Lee, who concerted efforts to form the MOU said, “This agreement will bring together two of the world’s leading brain-related research teams, and I hope that combining their expertise will bring great advances in brain science and engineering.
KAIST’s College of Life Science and Bioengineering, which is known for its creative interdisciplinary research, is producing exemplary research results in the field of brain science from its Biological Sciences and Bio and Brain Engineering departments.
In addition to cooperation with Harvard, KAIST has also formed partnerships with Emory University, Japan’s RIKEN Brain Institute, and Germany’s Max Planck Institute. Not only does it have a worldwide network pertaining to brain research, but KAIST has also engaged in cooperative research with prominent domestic institutions such as, Asan Medical Center, the Korea Research Institute of Bioscience and Biotechnology, the Korea Research Institute of Standards and Science, and the SK Corporation. Through these connections, KAIST has managed to lead in mutually cooperative brain interdisciplinary research.
2009.08.10 View 20890 -
 KAIST Signs Agreement for Industry-Academia Cooperation with KCC
KAIST signed an agreement for industry-academia cooperation with KCC, Korea"s leading supplier of building & industrial materials, on May 28, university sources said.
The agreement signed by KAIST President Nam-Pyo Suh and Mong-Jin Chung, Chairman of the KCC Business Group, calls for KAIST and KCC to conduct joint research for the development of new technologies in nano science, new materials areas and interdisciplinary areas.
Under the agreement, KCC will invest 5 billion won into the KAIST Institute for the NanoCentury over the next five years.
KCC Chairman Chung said: "Through this industry-academia cooperation agreement, we are seeking to give part of our profits back to community . We hope this agreement to contribute to the development of core technologies of the future in the new materials field, and nurturing specialized manpower."
2009.05.28 View 16453
KAIST Signs Agreement for Industry-Academia Cooperation with KCC
KAIST signed an agreement for industry-academia cooperation with KCC, Korea"s leading supplier of building & industrial materials, on May 28, university sources said.
The agreement signed by KAIST President Nam-Pyo Suh and Mong-Jin Chung, Chairman of the KCC Business Group, calls for KAIST and KCC to conduct joint research for the development of new technologies in nano science, new materials areas and interdisciplinary areas.
Under the agreement, KCC will invest 5 billion won into the KAIST Institute for the NanoCentury over the next five years.
KCC Chairman Chung said: "Through this industry-academia cooperation agreement, we are seeking to give part of our profits back to community . We hope this agreement to contribute to the development of core technologies of the future in the new materials field, and nurturing specialized manpower."
2009.05.28 View 16453 -
 KAIST Prof. Park Selected as Winner of Clemson Award
Professor Tae-Gwan Park of the Department of Biological Sciences, KAIST, was chosen as the winner of the 2009 Clemson Award for Fundamental Research, university authorities said on Tuesday (April 7).
The award is the highest recognition of the Society for Biomaterials, an international organization of more than 3,000 members that promotes research in the field of biomaterials. Prof. Park is cited for his outstanding achievements in interdisciplinary research covering gene transferring, gene therapy and neogenesis. It is rare for a non-U.S. national to win the prize in the 36-year history of the award.
The award will be given to Professor Park at the Annual Meeting of the society which will be held in San Antonio, Texas, on April 22.
2009.04.09 View 15314
KAIST Prof. Park Selected as Winner of Clemson Award
Professor Tae-Gwan Park of the Department of Biological Sciences, KAIST, was chosen as the winner of the 2009 Clemson Award for Fundamental Research, university authorities said on Tuesday (April 7).
The award is the highest recognition of the Society for Biomaterials, an international organization of more than 3,000 members that promotes research in the field of biomaterials. Prof. Park is cited for his outstanding achievements in interdisciplinary research covering gene transferring, gene therapy and neogenesis. It is rare for a non-U.S. national to win the prize in the 36-year history of the award.
The award will be given to Professor Park at the Annual Meeting of the society which will be held in San Antonio, Texas, on April 22.
2009.04.09 View 15314 -
 Prof. Song Develops Nano-Structure to Enhance Power of Rechargeable Lithium-ion Battery
A team of scientists led by Prof. Hyun-Joon Song of the Department of Chemistry, KAIST, developed a nano-structure that could increase the power of rechargeable lithium-ion batteries, university sources said on Monday (Feb. 16).
The research team found that a nano-structured material using copper oxide (CuO) could produce lithium-ion batteries with some 50 percent more capacity than conventional products. The study was published in the online edition of peer-review journal Advanced Materials.
In rechargeable lithium-ion batteries, lithium ions move between the battery"s anode and cathode. The high-energy density of the batteries led to their common use in consumer electronics products, expecially portable devices. Their demand in automotive and aerospace applications is growing, and nano-structured, or nano-enabled batteries are emerging as the new generation of lithium-ion batteries for their edge in recharging time, capacity and battery life.
Graphite has been a popular material for cathodes in lithium-ion batteries. However, graphite cathodes are also blamed for lost capacity due to their consumption of lithium ions, which are linked to shorter battery life.
As such, scientists have been looking for materials that could replace graphite in cathodes, and silicon and metal oxide have been studied as possible alternatives.
2009.02.17 View 14411
Prof. Song Develops Nano-Structure to Enhance Power of Rechargeable Lithium-ion Battery
A team of scientists led by Prof. Hyun-Joon Song of the Department of Chemistry, KAIST, developed a nano-structure that could increase the power of rechargeable lithium-ion batteries, university sources said on Monday (Feb. 16).
The research team found that a nano-structured material using copper oxide (CuO) could produce lithium-ion batteries with some 50 percent more capacity than conventional products. The study was published in the online edition of peer-review journal Advanced Materials.
In rechargeable lithium-ion batteries, lithium ions move between the battery"s anode and cathode. The high-energy density of the batteries led to their common use in consumer electronics products, expecially portable devices. Their demand in automotive and aerospace applications is growing, and nano-structured, or nano-enabled batteries are emerging as the new generation of lithium-ion batteries for their edge in recharging time, capacity and battery life.
Graphite has been a popular material for cathodes in lithium-ion batteries. However, graphite cathodes are also blamed for lost capacity due to their consumption of lithium ions, which are linked to shorter battery life.
As such, scientists have been looking for materials that could replace graphite in cathodes, and silicon and metal oxide have been studied as possible alternatives.
2009.02.17 View 14411 -
 KAIST Research Team Unveils Method to Fabricate Photonic Janus Balls
A research team led by Prof. Seung-Man Yang of the Department of Chemical and Biomolecular Engineering has found a method to fabricate photonic Janus balls with isotropic structural colors.
The finding draws attention since the newly-fabricated photonic balls may prove useful pigments for the realization of e-paper or flexible electronic displays.
The breakthrough was published in the Nov. 3 edition of the science journal "Advanced Materials." The Nov. 6 issue of "Nature" also featured it as one of the research highlights under the title of "Future Pixels."
Prof. Yang"s research team found that tiny marbles, black on one side and colored on the other, can be made by "curing" suspensions of silica particles with an ultraviolet lamp. When an electric field is applied, the marbles line up so that the black sides all face upwards, which suggests they may prove useful pigments for flexible electronic displays.
The researchers suspended a flow of carbon-black particles mixed with silica and a transparent or colored silica flow in a resin that polymerizes under ultraviolet light. They then passed the mixture through a tiny see-through tube. The light solidified the silica and resin as balls with differently colored regions, each about 200 micrometers in diameter.
Over the last decades, the development of industrial platforms to artificially fabricate structural color pigments has been a pressing issue in the research areas of materials science and optics. Prof. Yang, who is also the director of the National Creative Research Initiative Center for Integrated Optofluidic Systems, has led the researches focused on fabrication of functional nano-materials through the process of assembling nano-building blocks into designed patterns.
The "complementary hybridization of optical and fluidic devices for integrated optofluidic systems" research was supported by a grant from the Creative Research Initiative Program of the Ministry of Education, Science & Technology.
2008.11.12 View 18019
KAIST Research Team Unveils Method to Fabricate Photonic Janus Balls
A research team led by Prof. Seung-Man Yang of the Department of Chemical and Biomolecular Engineering has found a method to fabricate photonic Janus balls with isotropic structural colors.
The finding draws attention since the newly-fabricated photonic balls may prove useful pigments for the realization of e-paper or flexible electronic displays.
The breakthrough was published in the Nov. 3 edition of the science journal "Advanced Materials." The Nov. 6 issue of "Nature" also featured it as one of the research highlights under the title of "Future Pixels."
Prof. Yang"s research team found that tiny marbles, black on one side and colored on the other, can be made by "curing" suspensions of silica particles with an ultraviolet lamp. When an electric field is applied, the marbles line up so that the black sides all face upwards, which suggests they may prove useful pigments for flexible electronic displays.
The researchers suspended a flow of carbon-black particles mixed with silica and a transparent or colored silica flow in a resin that polymerizes under ultraviolet light. They then passed the mixture through a tiny see-through tube. The light solidified the silica and resin as balls with differently colored regions, each about 200 micrometers in diameter.
Over the last decades, the development of industrial platforms to artificially fabricate structural color pigments has been a pressing issue in the research areas of materials science and optics. Prof. Yang, who is also the director of the National Creative Research Initiative Center for Integrated Optofluidic Systems, has led the researches focused on fabrication of functional nano-materials through the process of assembling nano-building blocks into designed patterns.
The "complementary hybridization of optical and fluidic devices for integrated optofluidic systems" research was supported by a grant from the Creative Research Initiative Program of the Ministry of Education, Science & Technology.
2008.11.12 View 18019 -
 KAIST Team Identifies Nano-scale Origin of Toughness in Rare Earth-added Silicon Carbide
A research team led by Prof. Do-Kyung Kim of the Department of Materials Science and Engineering of KAIST has identified the nano-scale origin of the toughness in rare-earth doped silicon carbide (RE-SiC), university sources said on Monday (Oct. 6).
The research was conducted jointly with a U.S. team headed by Prof. R. O. Ritchie of the Department of Materials Science and Engineering, University of California, Berkeley.
The findings were carried in the online edition of Nano Letters published by the American Chemical Association.
Silicon carbide, a ceramic material known to be one of the hardest substances, are potential candidate materials for many ultrahigh-temperature structural applications. For example, if SiC, instead of metallic alloys, is used in gas-turbine engines for power generation and aerospace applications, operating temperatures of many hundred degrees higher can be obtained with a consequent dramatic increase in thermodynamic efficiency and reduced fuel consumption. However, the use of such ceramic materials has so far been severely limited since the origin of the toughness in RE-SiC remained unknown thus far.
In order to investigate the origin of the toughness in RE-SiC, the researchers attempted to examine the mechanistic nature of the cracking events, which they found to occur precisely along the interface between SiC grains and the nano-scale grain-boundary phase, by using ultrahigh-resolution transmission electron microscopy and atomic-scale spectroscopy. The research found that for optimal toughness, the relative elastic modulus across the grain-boundary phase and the interfacial fracture toughness are the most critical material parameters; both can be altered with appropriate choice of rare-earth elements.
In addition to identifying the nano-scale origin of the toughness in RE-SiC, the findings also contributed to precisely predicting how the use of various rare-earth elements lead to difference in toughness.
University sources said that the findings will significantly advance the date when RE-SiC will replace metallic alloys in gas-turbine engines for power generation and aerospace applications.
2008.10.08 View 19013
KAIST Team Identifies Nano-scale Origin of Toughness in Rare Earth-added Silicon Carbide
A research team led by Prof. Do-Kyung Kim of the Department of Materials Science and Engineering of KAIST has identified the nano-scale origin of the toughness in rare-earth doped silicon carbide (RE-SiC), university sources said on Monday (Oct. 6).
The research was conducted jointly with a U.S. team headed by Prof. R. O. Ritchie of the Department of Materials Science and Engineering, University of California, Berkeley.
The findings were carried in the online edition of Nano Letters published by the American Chemical Association.
Silicon carbide, a ceramic material known to be one of the hardest substances, are potential candidate materials for many ultrahigh-temperature structural applications. For example, if SiC, instead of metallic alloys, is used in gas-turbine engines for power generation and aerospace applications, operating temperatures of many hundred degrees higher can be obtained with a consequent dramatic increase in thermodynamic efficiency and reduced fuel consumption. However, the use of such ceramic materials has so far been severely limited since the origin of the toughness in RE-SiC remained unknown thus far.
In order to investigate the origin of the toughness in RE-SiC, the researchers attempted to examine the mechanistic nature of the cracking events, which they found to occur precisely along the interface between SiC grains and the nano-scale grain-boundary phase, by using ultrahigh-resolution transmission electron microscopy and atomic-scale spectroscopy. The research found that for optimal toughness, the relative elastic modulus across the grain-boundary phase and the interfacial fracture toughness are the most critical material parameters; both can be altered with appropriate choice of rare-earth elements.
In addition to identifying the nano-scale origin of the toughness in RE-SiC, the findings also contributed to precisely predicting how the use of various rare-earth elements lead to difference in toughness.
University sources said that the findings will significantly advance the date when RE-SiC will replace metallic alloys in gas-turbine engines for power generation and aerospace applications.
2008.10.08 View 19013 -
 Storing Stably Hydrogen Atoms in Icy Materials Discovered
KAIST, Aug. 8, 2008 -- A KAIST research team led by Prof. Huen Lee of the Department of Chemical & Biomolecular Engineering has discovered that icy organic hydrates, which contain small cages that can trap guest molecules, can be used to create and trap hydrogen atoms at higher temperatures.
The properties and reactions of single hydrogen atoms are of great scientific interest because of their inherent quantum mechanical behavior; experimentally, they can be generated and stabilized at very low temperatures (4 K) by high-energy irradiation of solid molecular hydrogen.
The finding was reported in the journal of American Chemical Society and featured in the "Editor"s Choice" in the July 11 issue of Science as a recent research highlight.
Hydrogen is a clean and sustainable form of energy that can be used in mobile and stationary applications. Hydrogen has the potential to solve several major challenges today: depletion of fossil fuels, poor air quality, and green house gas emissions.
However, the trapping of hydrogen atoms in crystalline solid matrix has never been attempted mainly because of experimental difficulties in identifying the generated hydrogen atoms with either spectroscopic or microscopic technique.
"To overcome the barriers and limitations of the existing storage approaches, we have continuously attempted to find the new hydrogen storage media such as icy powders and other related inclusion compounds," said Prof. Lee
The discovery follows the breakthrough concept Prof. Lee"s research team proposed in Nature in 2005 to use pure ice to capture and store hydrogen molecules. At moderate temperature and pressure conditions small guest molecules are entrapped in pure ice powders to form the mixed icy hydrate materials.
"Stable existence of single hydrogen molecule/radical in icy crystalline matrices may offer significant advantages in exploring hydrogen as a quantum medium because icy hydrogen hydrates can be formed at milder conditions when compared with pure solid hydrogen, which requires the ultra low temperature of 4.2 K," said Prof. Lee.
The novel design and synthesis of ionic and radicalized icy hydrates are expected to open a new field for inclusion chemistry and ice-based science and technology. Specifically, the fact that hydrogen atoms can be stably stored in icy materials might provide versatile and practical applications to energy devices including fuel cells, ice-induced reactions, and novel energy storage process, according to the KAIST professor.
2008.08.07 View 16399
Storing Stably Hydrogen Atoms in Icy Materials Discovered
KAIST, Aug. 8, 2008 -- A KAIST research team led by Prof. Huen Lee of the Department of Chemical & Biomolecular Engineering has discovered that icy organic hydrates, which contain small cages that can trap guest molecules, can be used to create and trap hydrogen atoms at higher temperatures.
The properties and reactions of single hydrogen atoms are of great scientific interest because of their inherent quantum mechanical behavior; experimentally, they can be generated and stabilized at very low temperatures (4 K) by high-energy irradiation of solid molecular hydrogen.
The finding was reported in the journal of American Chemical Society and featured in the "Editor"s Choice" in the July 11 issue of Science as a recent research highlight.
Hydrogen is a clean and sustainable form of energy that can be used in mobile and stationary applications. Hydrogen has the potential to solve several major challenges today: depletion of fossil fuels, poor air quality, and green house gas emissions.
However, the trapping of hydrogen atoms in crystalline solid matrix has never been attempted mainly because of experimental difficulties in identifying the generated hydrogen atoms with either spectroscopic or microscopic technique.
"To overcome the barriers and limitations of the existing storage approaches, we have continuously attempted to find the new hydrogen storage media such as icy powders and other related inclusion compounds," said Prof. Lee
The discovery follows the breakthrough concept Prof. Lee"s research team proposed in Nature in 2005 to use pure ice to capture and store hydrogen molecules. At moderate temperature and pressure conditions small guest molecules are entrapped in pure ice powders to form the mixed icy hydrate materials.
"Stable existence of single hydrogen molecule/radical in icy crystalline matrices may offer significant advantages in exploring hydrogen as a quantum medium because icy hydrogen hydrates can be formed at milder conditions when compared with pure solid hydrogen, which requires the ultra low temperature of 4.2 K," said Prof. Lee.
The novel design and synthesis of ionic and radicalized icy hydrates are expected to open a new field for inclusion chemistry and ice-based science and technology. Specifically, the fact that hydrogen atoms can be stably stored in icy materials might provide versatile and practical applications to energy devices including fuel cells, ice-induced reactions, and novel energy storage process, according to the KAIST professor.
2008.08.07 View 16399 -
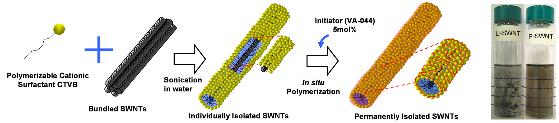 Research Outputs over Carbon Nanotube by Prof. Choi Selected as Research Highlight by ACS
Research Outputs over Carbon Nanotube by Prof. Choi Selected as Research Highlight by ACS
Research Outputs over Carbon Nanotube by Prof. Choi Selected as Research Highlight by ACS
A research team headed by Seong-Min Choi, a professor of Nuclear and Quantum Engineering, KAIST, has developed technologies to stably disperse carbon nanotube particles in aqueous solutions and organic solvents, essential for industrial applications of carbon nanotube, and discovered the dispersion characteristics of carbon nanotube. The research outputs have been published by ‘Advanced materials’ (19, 929, 2007), the most distinguished journal in Material Science field, and introduced as Research Highlight at the May 7th edition of ‘Heart Cut’ by the American Chemical Society (ACS).
A number of processes for industrial applications of carbon nanotube require the dispersion of carbon nanotube in aqueous solutions or organic solvents, and thus far, surfactant particles or DNAs have been used to disperse carbon nanotube particles. However, they have shortcomings of easy destruction of dispersion. In order to overcome such shortcomings, Prof. Choi’s team produced carbon nanotube particle-dispersed aqueous solutions by using surfactant particles and then polymerized surfactant particles absorbed to the surfaces of carbon nanotube in situ to develop carbon nanotube with hydrophile and safe surfaces. The functional carbon nanotube so obtained shows features of easy dispersion in aqueous solutions and organic solvents even after being processed, such as freeze drying, therefore, is expected to significantly contribute to the development of application technologies of carbon nanotubes. Tae-Hwan Kim and Chang-Woo Doh, both doctoral students, played key roles in the researches carried out under the auspices of the Ministry of Science and Technology (MOST) as a nuclear power R&D project, and the relevant technologies were filed for patent applications.
Figures: Carbon nanotube before polymerization (left), carbon nanotube polymerized with surfactant particles (right)
2007.05.14 View 16353
Research Outputs over Carbon Nanotube by Prof. Choi Selected as Research Highlight by ACS
Research Outputs over Carbon Nanotube by Prof. Choi Selected as Research Highlight by ACS
Research Outputs over Carbon Nanotube by Prof. Choi Selected as Research Highlight by ACS
A research team headed by Seong-Min Choi, a professor of Nuclear and Quantum Engineering, KAIST, has developed technologies to stably disperse carbon nanotube particles in aqueous solutions and organic solvents, essential for industrial applications of carbon nanotube, and discovered the dispersion characteristics of carbon nanotube. The research outputs have been published by ‘Advanced materials’ (19, 929, 2007), the most distinguished journal in Material Science field, and introduced as Research Highlight at the May 7th edition of ‘Heart Cut’ by the American Chemical Society (ACS).
A number of processes for industrial applications of carbon nanotube require the dispersion of carbon nanotube in aqueous solutions or organic solvents, and thus far, surfactant particles or DNAs have been used to disperse carbon nanotube particles. However, they have shortcomings of easy destruction of dispersion. In order to overcome such shortcomings, Prof. Choi’s team produced carbon nanotube particle-dispersed aqueous solutions by using surfactant particles and then polymerized surfactant particles absorbed to the surfaces of carbon nanotube in situ to develop carbon nanotube with hydrophile and safe surfaces. The functional carbon nanotube so obtained shows features of easy dispersion in aqueous solutions and organic solvents even after being processed, such as freeze drying, therefore, is expected to significantly contribute to the development of application technologies of carbon nanotubes. Tae-Hwan Kim and Chang-Woo Doh, both doctoral students, played key roles in the researches carried out under the auspices of the Ministry of Science and Technology (MOST) as a nuclear power R&D project, and the relevant technologies were filed for patent applications.
Figures: Carbon nanotube before polymerization (left), carbon nanotube polymerized with surfactant particles (right)
2007.05.14 View 16353